xxiii Ïανελληνιο ÏÏ Î½ÎµÎ´Ïιο ÏÏ ÏÎ¹ÎºÎ·Ï ÏÏεÏÎµÎ±Ï ÎºÎ±ÏαÏÏαÏÎ·Ï & εÏιÏÏÎ·Î¼Î·Ï ...
xxiii Ïανελληνιο ÏÏ Î½ÎµÎ´Ïιο ÏÏ ÏÎ¹ÎºÎ·Ï ÏÏεÏÎµÎ±Ï ÎºÎ±ÏαÏÏαÏÎ·Ï & εÏιÏÏÎ·Î¼Î·Ï ...
xxiii Ïανελληνιο ÏÏ Î½ÎµÎ´Ïιο ÏÏ ÏÎ¹ÎºÎ·Ï ÏÏεÏÎµÎ±Ï ÎºÎ±ÏαÏÏαÏÎ·Ï & εÏιÏÏÎ·Î¼Î·Ï ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Synthesis of Polymer Brushes onto Inorganic Nanoparticles<br />
D. S. Achilleos, M. Vamvakaki<br />
Institute of Electronic Structure and Laser, Foundation for Research and Technology - Hellas,<br />
711 10 Heraklion, Crete, Greece and<br />
Department of Materials Science and Technology, University of Crete, 710 03 Heraklion, Crete, Greece<br />
*achill@iesl.forth.gr; vamvakak@materials.uoc.gr<br />
Nanoparticles have attracted much attention due to their fascinating electronic, optical, magnetic, and/or catalytic<br />
properties associated with their nano- or quantum-scale dimensions. These include metal (Au, Pt, Pd, etc.), semiconductor<br />
(CdS, CdSe, ZnS, etc.), and oxide (Fe 2 O 3 , Al 2 O 3 , TiO 2 , SiO 2 , etc.) nanoparticles [1]. The development of polymer/nanoparticle<br />
composite materials is of greater interest due to the combination of both the properties of the inorganic nanoparticles and those<br />
of the polymer (solubility, film formation, and chemical activity) [2]. Although the surface modification of silica particles by<br />
the chemical attachment of polymer chains has been extensively reported [3], other particles, like CdS [4], Fe 2 O 3 [5] and TiO 2<br />
[6] have been also used. However, so far there are no reports on the synthesis of chemically bound polymer/ZnO hybrids.<br />
In the present work we describe the preparation of smart surface polymeric coatings onto inorganic nanoparticles.<br />
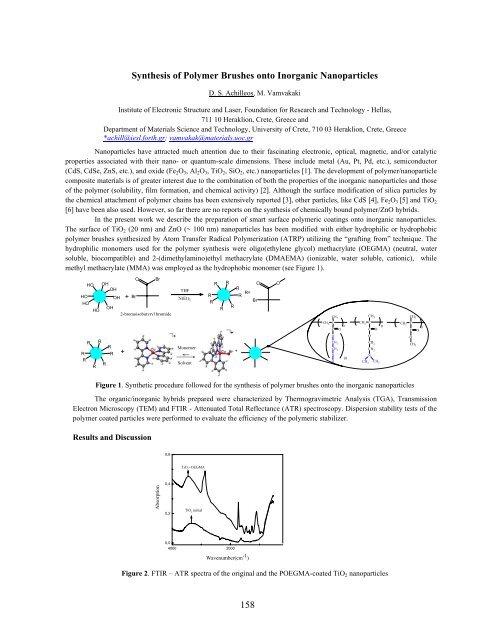
The surface of TiO 2 (20 nm) and ZnO (~ 100 nm) nanoparticles has been modified with either hydrophilic or hydrophobic<br />
polymer brushes synthesized by Atom Transfer Radical Polymerization (ATRP) utilizing the “grafting from” technique. The<br />
hydrophilic monomers used for the polymer synthesis were oligo(ethylene glycol) methacrylate (OEGMA) (neutral, water<br />
soluble, biocompatible) and 2-(dimethylamino)ethyl methacrylate (DMAEMA) (ionizable, water soluble, cationic), while<br />
methyl methacrylate (MMA) was employed as the hydrophobic monomer (see Figure 1).<br />
O Br<br />
HO OH<br />
HO<br />
OH<br />
OH + Br<br />
HO<br />
HO<br />
OH<br />
2-bromoisobutyryl bromide<br />
R<br />
R<br />
R<br />
R<br />
R<br />
R<br />
R<br />
R<br />
+<br />
THF<br />
N(Et) 3<br />
Monomer<br />
Solvent<br />
R R<br />
O<br />
R<br />
R R R=<br />
R<br />
R<br />
R<br />
+<br />
Br<br />
O<br />
CH 3<br />
CH 2 C<br />
n<br />
C O<br />
O<br />
CH 2<br />
CH 2<br />
O<br />
10<br />
H<br />
CH 3<br />
CH 2 C<br />
CH 3<br />
CH 2 C<br />
C O<br />
n<br />
C O<br />
CH 3<br />
O<br />
CH 2<br />
O<br />
CH 3<br />
CH 2<br />
N<br />
Figure 1. Synthetic procedure followed for the synthesis of polymer brushes onto the inorganic nanoparticles<br />
The organic/inorganic hybrids prepared were characterized by Thermogravimetric Analysis (TGA), Transmission<br />
Electron Microscopy (TEM) and FTIR - Attenuated Total Reflectance (ATR) spectroscopy. Dispersion stability tests of the<br />
polymer coated particles were performed to evaluate the efficiency of the polymeric stabilizer.<br />
Results and Discussion<br />
0,6<br />
TiO 2 +OEGMA<br />
Absorption<br />
0,4<br />
0,2<br />
TiO 2 initial<br />
0,0<br />
4000 2000<br />
Wavenumber(cm -1 )<br />
Figure 2. FTIR – ATR spectra of the original and the POEGMA-coated TiO 2 nanoparticles<br />
158