xxiii Ïανελληνιο ÏÏ Î½ÎµÎ´Ïιο ÏÏ ÏÎ¹ÎºÎ·Ï ÏÏεÏÎµÎ±Ï ÎºÎ±ÏαÏÏαÏÎ·Ï & εÏιÏÏÎ·Î¼Î·Ï ...
xxiii Ïανελληνιο ÏÏ Î½ÎµÎ´Ïιο ÏÏ ÏÎ¹ÎºÎ·Ï ÏÏεÏÎµÎ±Ï ÎºÎ±ÏαÏÏαÏÎ·Ï & εÏιÏÏÎ·Î¼Î·Ï ...
xxiii Ïανελληνιο ÏÏ Î½ÎµÎ´Ïιο ÏÏ ÏÎ¹ÎºÎ·Ï ÏÏεÏÎµÎ±Ï ÎºÎ±ÏαÏÏαÏÎ·Ï & εÏιÏÏÎ·Î¼Î·Ï ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
AP-PH (a.u.)<br />
0.4<br />
0.3<br />
0.2<br />
0.1<br />
0.0<br />
0 5 10 15 20 25 30 35 40 45<br />
Ba deposition (min)<br />
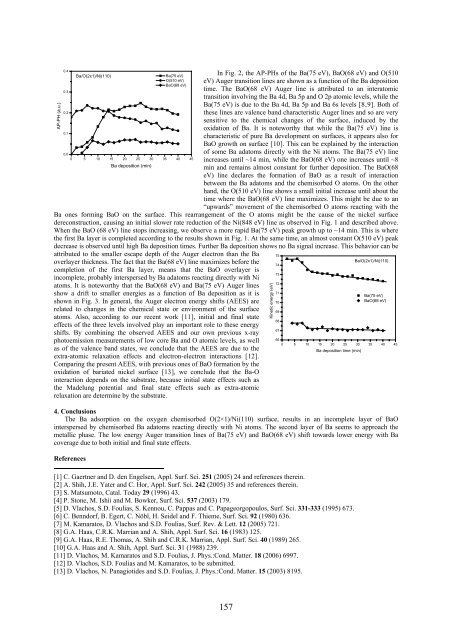
Figure 2 The AP-PHs of the Ba(75 eV), BaO(68<br />
eV) and O(510 eV) Auger transition lines as a<br />
function of the Ba deposition on the oxygen<br />
chemisorbed phase O(2×1)/Ni(110).<br />
In Fig. 2, the AP-PHs of the Ba(75 eV), BaO(68 eV) and O(510<br />
eV) Auger transition lines are shown as a function of the Ba deposition<br />
time. The BaO(68 eV) Auger line is attributed to an interatomic<br />
transition involving the Ba 4d, Ba 5p and O 2p atomic levels, while the<br />
Ba(75 eV) is due to the Ba 4d, Ba 5p and Ba 6s levels [8,9]. Both of<br />
these lines are valence band characteristic Auger lines and so are very<br />
sensitive to the chemical changes of the surface, induced by the<br />
oxidation of Ba. It is noteworthy that while the Ba(75 eV) line is<br />
characteristic of pure Ba development on surfaces, it appears also for<br />
BaO growth on surface [10]. This can be explained by the interaction<br />
of some Ba adatoms directly with the Ni atoms. The Ba(75 eV) line<br />
increases until ~14 min, while the BaO(68 eV) one increases until ~8<br />
min and remains almost constant for further deposition. The BaO(68<br />
eV) line declares the formation of BaO as a result of interaction<br />
between the Ba adatoms and the chemisorbed O atoms. On the other<br />
hand, the O(510 eV) line shows a small initial increase until about the<br />
time where the BaO(68 eV) line maximizes. This might be due to an<br />
“upwards” movement of the chemisorbed O atoms reacting with the<br />
Ba ones forming BaO on the surface. This rearrangement of the O atoms might be the cause of the nickel surface<br />
dereconstruction, causing an initial slower rate reduction of the Ni(848 eV) line as observed in Fig. 1 and described above.<br />
When the BaO (68 eV) line stops increasing, we observe a more rapid Ba(75 eV) peak growth up to ~14 min. This is where<br />
the first Ba layer is completed according to the results shown in Fig. 1. At the same time, an almost constant O(510 eV) peak<br />
decrease is observed until high Ba deposition times. Further Ba deposition shows no Ba signal increase. This behavior can be<br />
attributed to the smaller escape depth of the Auger electron than the Ba<br />
overlayer thickness. The fact that the Ba(68 eV) line maximizes before the<br />
completion of the first Ba layer, means that the BaO overlayer is<br />
incomplete, probably interspersed by Ba adatoms reacting directly with Ni<br />
atoms. It is noteworthy that the BaO(68 eV) and Ba(75 eV) Auger lines<br />
show a drift to smaller energies as a function of Ba deposition as it is<br />
shown in Fig. 3. In general, the Auger electron energy shifts (AEES) are<br />
related to changes in the chemical state or environment of the surface<br />
atoms. Also, according to our recent work [11], initial and final state<br />
effects of the three levels involved play an important role to these energy<br />
shifts. By combining the observed AEES and our own previous x-ray<br />
photoemission measurements of low core Ba and O atomic levels, as well<br />
as of the valence band states, we conclude that the AEES are due to the<br />
extra-atomic relaxation effects and electron-electron interactions [12].<br />
Comparing the present AEES, with previous ones of BaO formation by the<br />
oxidation of bariated nickel surface [13], we conclude that the Ba-O<br />
interaction depends on the substrate, because initial state effects such as<br />
the Madelung potential and final state effects such as extra-atomic<br />
relaxation are determine by the substrate.<br />
4. Conclusions<br />
The Ba adsorption on the oxygen chemisorbed O(2×1)/Ni(110) surface, results in an incomplete layer of BaO<br />
interspersed by chemisorbed Ba adatoms reacting directly with Ni atoms. The second layer of Ba seems to approach the<br />
metallic phase. The low energy Auger transition lines of Ba(75 eV) and BaO(68 eV) shift towards lower energy with Ba<br />
coverage due to both initial and final state effects.<br />
References<br />
Ba/O(2x1)/Ni(110)<br />
Ba(75 eV)<br />
O(510 eV)<br />
BaO(68 eV)<br />
66<br />
0 5 10 15 20 25 30 35 40 45<br />
Figure 3 The energy of the Ba(73 eV) and<br />
BaO(68 eV) Auger transition lines for Ba<br />
deposition on the oxygen chemisorbed phase<br />
O(2×1)/Ni(110).<br />
[1] C. Gaertner and D. den Engelsen, Appl. Surf. Sci. 251 (2005) 24 and references therein.<br />
[2] A. Shih, J.E. Yater and C. Hor, Appl. Surf. Sci. 242 (2005) 35 and references therein.<br />
[3] S. Matsumoto, Catal. Today 29 (1996) 43.<br />
[4] P. Stone, M. Ishii and M. Bowker, Surf. Sci. 537 (2003) 179.<br />
[5] D. Vlachos, S.D. Foulias, S. Kennou, C. Pappas and C. Papageorgopoulos, Surf. Sci. 331-333 (1995) 673.<br />
[6] C. Benndorf, B. Egert, C. Nöbl, H. Seidel and F. Thieme, Surf. Sci. 92 (1980) 636.<br />
[7] M. Kamaratos, D. Vlachos and S.D. Foulias, Surf. Rev. & Lett. 12 (2005) 721.<br />
[8] G.A. Haas, C.R.K. Marrian and A. Shih, Appl. Surf. Sci. 16 (1983) 125.<br />
[9] G.A. Haas, R.E. Thomas, A. Shih and C.R.K. Marrian, Appl. Surf. Sci. 40 (1989) 265.<br />
[10] G.A. Haas and A. Shih, Appl. Surf. Sci. 31 (1988) 239.<br />
[11] D. Vlachos, M. Kamaratos and S.D. Foulias, J. Phys.:Cond. Matter. 18 (2006) 6997.<br />
[12] D. Vlachos, S.D. Foulias and M. Kamaratos, to be submitted.<br />
[13] D. Vlachos, N. Panagiotides and S.D. Foulias, J. Phys.:Cond. Matter. 15 (2003) 8195.<br />
Kinetic energy (eV)<br />
75<br />
74<br />
73<br />
72<br />
71<br />
70<br />
69<br />
68<br />
67<br />
Ba deposition time (min)<br />
Ba/O(2x1)/Ni(110)<br />
Ba(75 eV)<br />
BaO(68 eV)<br />
157