xxiii Ïανελληνιο ÏÏ Î½ÎµÎ´Ïιο ÏÏ ÏÎ¹ÎºÎ·Ï ÏÏεÏÎµÎ±Ï ÎºÎ±ÏαÏÏαÏÎ·Ï & εÏιÏÏÎ·Î¼Î·Ï ...
xxiii Ïανελληνιο ÏÏ Î½ÎµÎ´Ïιο ÏÏ ÏÎ¹ÎºÎ·Ï ÏÏεÏÎµÎ±Ï ÎºÎ±ÏαÏÏαÏÎ·Ï & εÏιÏÏÎ·Î¼Î·Ï ...
xxiii Ïανελληνιο ÏÏ Î½ÎµÎ´Ïιο ÏÏ ÏÎ¹ÎºÎ·Ï ÏÏεÏÎµÎ±Ï ÎºÎ±ÏαÏÏαÏÎ·Ï & εÏιÏÏÎ·Î¼Î·Ï ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
diamond, there are identified graphite and carbon, while the amorphous material is also significant (26%). The diamond peaks<br />
show a significant base (2theta) which means that the structure of this phase is probably crypocrystallic and also are symmetrical<br />
which means that the diamond phase is well crystallized.<br />
The BG/ND sample presents two strong peaks at about 10-13 o 2theta and 25-37 o 2theta, while there are two less intense<br />
peaks at about 17-24 o 2theta and 28-55 o 2theta. Significantly, the first peak (10-13 o 2theta) is less intense to the pure BG sample,<br />
which indicates a possible transition from the amorphous state to a primary amorphous-cryptocrystallic state. Additionally, the<br />
amorphous part of the pure ND sample affects the total amount and the morphology of amorphous in the BG/ND sample, while<br />
the peak between 28-55 o 2theta can be attributed to the cryptocrystallic structure of diamond that appears between 43-44 ο 2theta.<br />
Amorphous<br />
SCS: Sodium Calcium Silicate, Ca SiO<br />
86-0399 ICDD card<br />
D: Diamond, C, 06-0675 ICDD card<br />
G: Graphite, C, 75-2078 ICDD card<br />
C: Carbon, C, 26-1069 ICDD card<br />
C<br />
G<br />
ND pure<br />
D<br />
SCS<br />
SCS<br />
5 15 25 35 45 55 65 75<br />
SCS<br />
Amorphous<br />
SCS<br />
SCS BG/ND<br />
SCS SCS<br />
SCS SCS SCS<br />
SCS<br />
SCS<br />
5 10 15 20 25 30 35 40 45 50 55 60 65 70 75 BG pure 80<br />
Amorphous<br />
5 10 15 20 25 30 35 40 45 50 55 60 65 70 75 80<br />
D<br />
Fig. 1. XRD patterns of the BG/ND system<br />
and of pure BG and ND<br />
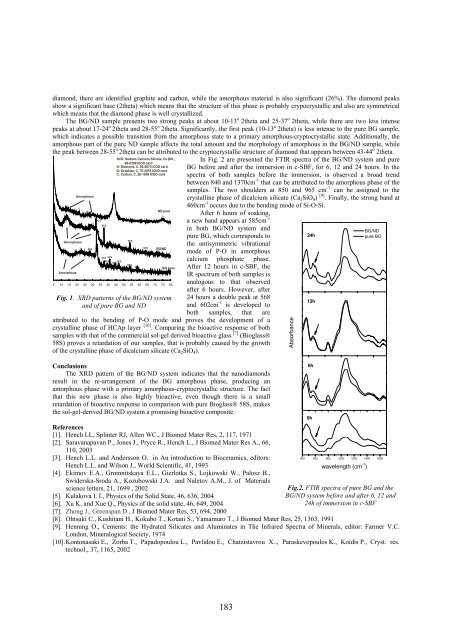
In Fig. 2 are presented the FTIR spectra of the BG/ND system and pure<br />
BG before and after the immersion in c-SBF, for 6, 12 and 24 hours. In the<br />
spectra of both samples before the immersion, is observed a broad trend<br />
between 840 and 1370cm -1 that can be attributed to the amorphous phase of the<br />
samples. The two shoulders at 850 and 965 cm -1 can be assigned to the<br />
crystalline phase of dicalcium silicate (Ca 2 SiO 4 ) [9] . Finally, the strong band at<br />
460cm -1 occurs due to the bending mode of Si-O-Si.<br />
After 6 hours of soaking,<br />
a new band appears at 585cm -1<br />
in both BG/ND system and<br />
pure BG, which corresponds to<br />
the antisymmetric vibrational<br />
mode of P-O in amorphous<br />
calcium phosphate phase.<br />
After 12 hours in c-SBF, the<br />
IR spectrum of both samples is<br />
analogous to that observed<br />
after 6 hours. However, after<br />
24 hours a double peak at 568<br />
and 602cm -1 is developed to<br />
both samples, that are<br />
attributed to the bending of P-O mode and proves the development of a<br />
crystalline phase of HCAp layer [10] . Comparing the bioactive response of both<br />
samples with that of the commercial sol-gel derived bioactive glass [7] (Bioglass®<br />
58S) proves a retardation of our samples, that is probably caused by the growth<br />
of the crystalline phase of dicalcium silicate (Ca 2 SiO 4 ).<br />
Conclusions<br />
The XRD pattern of the BG/ND system indicates that the nanodiamonds<br />
result in the re-arrangement of the BG amorphous phase, producing an<br />
amorphous phase with a primary amorphous-cryptocrystallic structure. The fact<br />
that this new phase is also highly bioactive, even though there is a small<br />
retardation of bioactive response in comparison with pure Bioglass® 58S, makes<br />
the sol-gel-derived BG/ND system a promising bioactive composite.<br />
2 4<br />
References<br />
[1]. Hench LL, Splinter RJ, Allen WC., J Biomed Mater Res, 2, 117, 1971<br />
[2]. Saravanapavan P., Jones J., Pryce R., Hench L., J Biomed Mater Res A., 66,<br />
110, 2003<br />
[3]. Hench L.L. and Andersson O. in An introduction to Bioceramics, editors:<br />
Hench L.L. and Wilson J., World Scientific, 41, 1993<br />
[4]. Ekimov E.A., Gromnitskaya E.L., Gierlotka S., Lojkowski W., Palosz B.,<br />
Swiderska-Sroda A., Kozubowski J.A. and Naletov A.M., J. of Materials<br />
science letters, 21, 1699 , 2002<br />
[5]. Kulakova I. I., Physics of the Solid State, 46, 636, 2004<br />
[6]. Xu K. and Xue Q., Physics of the solid state, 46, 649, 2004<br />
[7]. Zhong J., Greenspan D., J Biomed Mater Res, 53, 694, 2000<br />
[8]. Ohtsuki C., Kushitani H., Kokubo T., Kotani S., Yamamuro T., J Biomed Mater Res, 25, 1363, 1991<br />
400 600 800 1000 1200 1400 1600<br />
[9]. Henning O., Cements: the Hydrated Silicates and Aluminates in The Infrared Spectra of Minerals, editor: Farmer V.C.<br />
London, Mineralogical Society, 1974<br />
[10]. Kontonasaki E., Zorba T., Papadopoulou L., Pavlidou E., Chatzistavrou X.., Paraskevopoulos K., Koidis P., Cryst. res.<br />
technol., 37, 1165, 2002<br />
Absorbance<br />
24h<br />
12h<br />
6h<br />
0h<br />
wavelength (cm -1 )<br />
BG/ND<br />
pure BG<br />
Fig.2. FTIR spectra of pure BG and the<br />
BG/ND system before and after 6, 12 and<br />
24h of immersion in c-SBF<br />
183