xxiii Ïανελληνιο ÏÏ Î½ÎµÎ´Ïιο ÏÏ ÏÎ¹ÎºÎ·Ï ÏÏεÏÎµÎ±Ï ÎºÎ±ÏαÏÏαÏÎ·Ï & εÏιÏÏÎ·Î¼Î·Ï ...
xxiii Ïανελληνιο ÏÏ Î½ÎµÎ´Ïιο ÏÏ ÏÎ¹ÎºÎ·Ï ÏÏεÏÎµÎ±Ï ÎºÎ±ÏαÏÏαÏÎ·Ï & εÏιÏÏÎ·Î¼Î·Ï ...
xxiii Ïανελληνιο ÏÏ Î½ÎµÎ´Ïιο ÏÏ ÏÎ¹ÎºÎ·Ï ÏÏεÏÎµÎ±Ï ÎºÎ±ÏαÏÏαÏÎ·Ï & εÏιÏÏÎ·Î¼Î·Ï ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Templated Sol-Gel Synthesis Of TiO 2 Nanoparticles<br />
Presenting Nanowire-Like Structure For Dye-Sensitized<br />
Solar Cells<br />
N. Alexaki, T. Stergiopoulos, A. G. Kontos, D. Tsoukleris, P. Falaras<br />
Institute of Physical Chemistry, NCSR Demokritos, 15310 Aghia Paraskevi Attikis, Athens, Greece<br />
*papi@chem.demokritos.gr<br />
Several works have been directed toward optimizing the efficiency of dye-sensitized solar cells (DSSCs) by developing<br />
novel methods of nanotitania synthesis and deposition on TCO. Functional properties of TiO 2 are influenced by many<br />
factors such as crystallinity, particle size and surface area and all these parameters are related to different conditions of<br />
preparation. Very recently, this work has been focused on preparing nanoparticles in the form of wires and tubes [1].<br />
Sol-gel process is favoured as an appropriate way for preparing nanomaterials because by the correct<br />
template choice, alkoxide precursor concentration, hydrolysis-condensation reaction conditions and sintering<br />
temperature important parameters such as particle size, film thickness and porosity can be readily controlled [2].<br />
Nanocrystalline titania was synthesized by a sol-gel method using tetraisopropyl orthotitanate (TIPT) modified with<br />
acetylacetone (ACA)/alkylamine hydrochloride [3]. In this work, results with hexadecylamine-based synthesis are<br />
reported.<br />
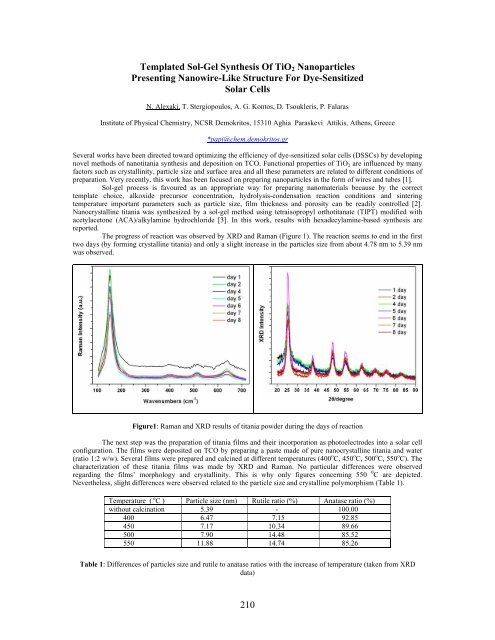
The progress of reaction was observed by XRD and Raman (Figure 1). The reaction seems to end in the first<br />
two days (by forming crystalline titania) and only a slight increase in the particles size from about 4.78 nm to 5.39 nm<br />
was observed.<br />
Figure1: Raman and XRD results of titania powder during the days of reaction<br />
The next step was the preparation of titania films and their incorporation as photoelectrodes into a solar cell<br />
configuration. The films were deposited on TCO by preparing a paste made of pure nanocrystalline titania and water<br />
(ratio 1:2 w/w). Several films were prepared and calcined at different temperatures (400 o C, 450 o C, 500 o C, 550 o C). The<br />
characterization of these titania films was made by XRD and Raman. No particular differences were observed<br />
regarding the films’ morphology and crystallinity. This is why only figures concerning 550 0 C are depicted.<br />
Nevertheless, slight differences were observed related to the particle size and crystalline polymorphism (Table 1).<br />
Temperature ( o C ) Particle size (nm) Rutile ratio (%) Anatase ratio (%)<br />
without calcination 5.39 - 100.00<br />
400 6.47 7.15 92.85<br />
450 7.17 10.34 89.66<br />
500 7.90 14.48 85.52<br />
550 11.88 14.74 85.26<br />
Table 1: Differences of particles size and rutile to anatase ratios with the increase of temperature (taken from XRD<br />
data)<br />
210