xxiii Ïανελληνιο ÏÏ Î½ÎµÎ´Ïιο ÏÏ ÏÎ¹ÎºÎ·Ï ÏÏεÏÎµÎ±Ï ÎºÎ±ÏαÏÏαÏÎ·Ï & εÏιÏÏÎ·Î¼Î·Ï ...
xxiii Ïανελληνιο ÏÏ Î½ÎµÎ´Ïιο ÏÏ ÏÎ¹ÎºÎ·Ï ÏÏεÏÎµÎ±Ï ÎºÎ±ÏαÏÏαÏÎ·Ï & εÏιÏÏÎ·Î¼Î·Ï ...
xxiii Ïανελληνιο ÏÏ Î½ÎµÎ´Ïιο ÏÏ ÏÎ¹ÎºÎ·Ï ÏÏεÏÎµÎ±Ï ÎºÎ±ÏαÏÏαÏÎ·Ï & εÏιÏÏÎ·Î¼Î·Ï ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
the cement-perlite matrix and the oxide which adds nucleation sites for the hydration process. The shorter relaxation time of<br />
the samples containing TiO 2 in the hardening period suggests that the introduction of the oxide results in a denser pore<br />
structure with smaller crystallites.<br />
In comparison to the PC and the CP+PC samples, the EP+PC and EP+PC+TiO 2 ones show significantly increased T 1 in values<br />
due to the increased amount of water in the mixture (see Table 1). Also the initial period is characterized by almost stable T 1<br />
values and an abrupt starting of the acceleration period. This behaviour is probably related to the porous structure of the EP<br />
which tends to adsorb a high percentage of water that is stored inside and is going to react with the cement gel during the<br />
acceleration period. Comparing to the pure PC, the acceleration period of the EP+PC sample is severely prolonged. The<br />
addition of the oxide (PC+EP+TiO 2 ) reduces the acceleration period duration while the hydration graph stabilizes the step<br />
like form. This resembles the corresponding influence of the TiO 2 in the PC+TiO 2 and CP+PC+TiO 2 samples. On the other<br />
side, the T 1 fin values of both the EP+PC and EP+PC+TiO 2 samples remain bigger than that of the unmodified PC. This is<br />
related to the bigger size of the pores introduced by the expanded perlite in the modified cement matrix which is also<br />
responsible for the light concrete properties on the one side and the deterioration of the mechanical strength on the other.<br />
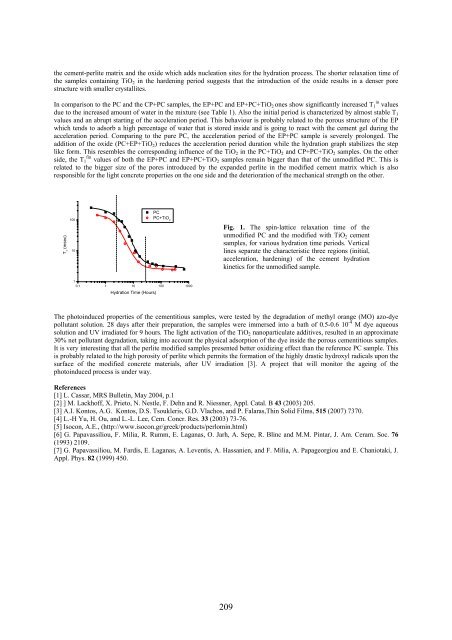
T 1<br />
(msec)<br />
100<br />
10<br />
PC<br />
PC+TiO 2<br />
Fig. 1. The spin-lattice relaxation time of the<br />
unmodified PC and the modified with TiO 2 cement<br />
samples, for various hydration time periods. Vertical<br />
lines separate the characteristic three regions (initial,<br />
acceleration, hardening) of the cement hydration<br />
kinetics for the unmodified sample.<br />
1<br />
0.1 1 10 100 1000<br />
Hydration Time (Hours)<br />
The photoinduced properties of the cementitious samples, were tested by the degradation of methyl orange (MO) azo-dye<br />
pollutant solution. 28 days after their preparation, the samples were immersed into a bath of 0.5-0.6 10 -4 M dye aqueous<br />
solution and UV irradiated for 9 hours. The light activation of the TiO 2 nanoparticulate additives, resulted in an approximate<br />
30% net pollutant degradation, taking into account the physical adsorption of the dye inside the porous cementitious samples.<br />
It is very interesting that all the perlite modified samples presented better oxidizing effect than the reference PC sample. This<br />
is probably related to the high porosity of perlite which permits the formation of the highly drastic hydroxyl radicals upon the<br />
surface of the modified concrete materials, after UV irradiation [3]. A project that will monitor the ageing of the<br />
photoinduced process is under way.<br />
References<br />
[1] L. Cassar, MRS Bulletin, May 2004, p.1<br />
[2] ] M. Lackhoff, X. Prieto, N. Nestle, F. Dehn and R. Niessner, Appl. Catal. B 43 (2003) 205.<br />
[3] A.I. Kontos, A.G. Kontos, D.S. Tsoukleris, G.D. Vlachos, and P. Falaras,Thin Solid Films, 515 (2007) 7370.<br />
[4] L.-H Yu, H. Ou, and L.-L. Lee, Cem. Concr. Res. 33 (2003) 73-76.<br />
[5] Isocon, A.E., (http://www.isocon.gr/greek/products/perlomin.html)<br />
[6] G. Papavassiliou, F. Milia, R. Rumm, E. Laganas, O. Jarh, A. Sepe, R. Blinc and M.M. Pintar, J. Am. Ceram. Soc. 76<br />
(1993) 2109.<br />
[7] G. Papavassiliou, M. Fardis, E. Laganas, A. Leventis, A. Hassanien, and F. Milia, A. Papageorgiou and E. Chaniotaki, J.<br />
Appl. Phys. 82 (1999) 450.<br />
209