xxiii Ïανελληνιο ÏÏ Î½ÎµÎ´Ïιο ÏÏ ÏÎ¹ÎºÎ·Ï ÏÏεÏÎµÎ±Ï ÎºÎ±ÏαÏÏαÏÎ·Ï & εÏιÏÏÎ·Î¼Î·Ï ...
xxiii Ïανελληνιο ÏÏ Î½ÎµÎ´Ïιο ÏÏ ÏÎ¹ÎºÎ·Ï ÏÏεÏÎµÎ±Ï ÎºÎ±ÏαÏÏαÏÎ·Ï & εÏιÏÏÎ·Î¼Î·Ï ...
xxiii Ïανελληνιο ÏÏ Î½ÎµÎ´Ïιο ÏÏ ÏÎ¹ÎºÎ·Ï ÏÏεÏÎµÎ±Ï ÎºÎ±ÏαÏÏαÏÎ·Ï & εÏιÏÏÎ·Î¼Î·Ï ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
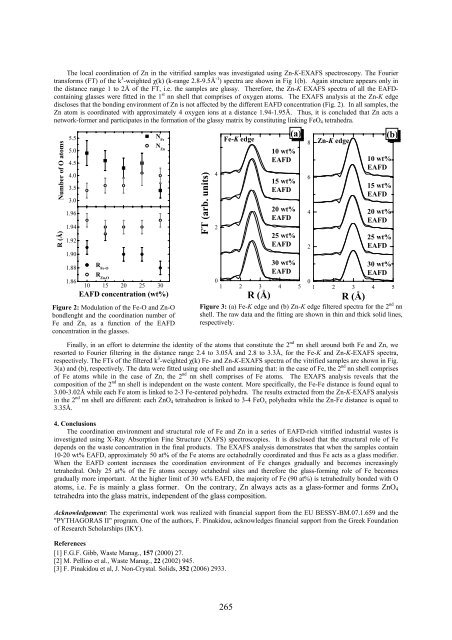
The local coordination of Zn in the vitrified samples was investigated using Zn-K-EXAFS spectroscopy. The Fourier<br />
transforms (FT) of the k 3 -weighted χ(k) (k-range 2.8-9.5Å -1 ) spectra are shown in Fig 1(b). Again structure appears only in<br />
the distance range 1 to 2Å of the FT, i.e. the samples are glassy. Therefore, the Zn-K EXAFS spectra of all the EAFDcontaining<br />
glasses were fitted in the 1 st nn shell that comprises of oxygen atoms. The EXAFS analysis at the Zn-K edge<br />
discloses that the bonding environment of Zn is not affected by the different EAFD concentration (Fig. 2). In all samples, the<br />
Zn atom is coordinated with approximately 4 oxygen ions at a distance 1.94-1.95Å. Thus, it is concluded that Zn acts a<br />
network-former and participates in the formation of the glassy matrix by constituting linking FeO 4 tetrahedra.<br />
Number of O atoms<br />
R (Å)<br />
5.5<br />
5.0<br />
4.5<br />
4.0<br />
3.5<br />
3.0<br />
1.96<br />
1.94<br />
1.92<br />
1.90<br />
1.88<br />
1.86<br />
R Fe-O<br />
R Zn-O<br />
N Fe<br />
N Zn<br />
10 15 20 25 30<br />
EAFD concentration (wt%)<br />
Figure 2: Modulation of the Fe-O and Zn-O<br />
bondlenght and the coordination number of<br />
Fe and Zn, as a function of the EAFD<br />
concentration in the glasses.<br />
FT (arb. units)<br />
4<br />
2<br />
Fe-K edge<br />
10 wt%<br />
EAFD<br />
15 wt%<br />
EAFD<br />
20 wt%<br />
EAFD<br />
25 wt%<br />
EAFD<br />
30 wt%<br />
EAFD<br />
0<br />
1 2 3 4 5<br />
R (Å)<br />
(a)<br />
8 Zn-K edge<br />
6<br />
4<br />
2<br />
10 wt%<br />
EAFD<br />
15 wt%<br />
EAFD<br />
20 wt%<br />
EAFD<br />
25 wt%<br />
EAFD<br />
30 wt%<br />
EAFD<br />
0<br />
1 2 3 4 5<br />
R (Å)<br />
(b)<br />
Figure 3: (a) Fe-K edge and (b) Zn-K edge filtered spectra for the 2 nd nn<br />
shell. The raw data and the fitting are shown in thin and thick solid lines,<br />
respectively.<br />
Finally, in an effort to determine the identity of the atoms that constitute the 2 nd nn shell around both Fe and Zn, we<br />
resorted to Fourier filtering in the distance range 2.4 to 3.05Å and 2.8 to 3.3Å, for the Fe-K and Zn-K-EXAFS spectra,<br />
respectively. The FTs of the filtered k 3 -weighted χ(k) Fe- and Zn-K-EXAFS spectra of the vitrified samples are shown in Fig.<br />
3(a) and (b), respectively. The data were fitted using one shell and assuming that: in the case of Fe, the 2 nd nn shell comprises<br />
of Fe atoms while in the case of Zn, the 2 nd nn shell comprises of Fe atoms. The EXAFS analysis reveals that the<br />
composition of the 2 nd nn shell is independent on the waste content. More specifically, the Fe-Fe distance is found equal to<br />
3.00-3.02Å while each Fe atom is linked to 2-3 Fe-centered polyhedra. The results extracted from the Zn-K-EXAFS analysis<br />
in the 2 nd nn shell are different: each ZnO 4 tetrahedron is linked to 3-4 FeO x polyhedra while the Zn-Fe distance is equal to<br />
3.35Å.<br />
4. Conclusions<br />
The coordination environment and structural role of Fe and Zn in a series of EAFD-rich vitrified industrial wastes is<br />
investigated using X-Ray Absorption Fine Structure (XAFS) spectroscopies. It is disclosed that the structural role of Fe<br />
depends on the waste concentration in the final products. The EXAFS analysis demonstrates that when the samples contain<br />
10-20 wt% EAFD, approximately 50 at% of the Fe atoms are octahedrally coordinated and thus Fe acts as a glass modifier.<br />
When the EAFD content increases the coordination environment of Fe changes gradually and becomes increasingly<br />
tetrahedral. Only 25 at% of the Fe atoms occupy octahedral sites and therefore the glass-forming role of Fe becomes<br />
gradually more important. At the higher limit of 30 wt% EAFD, the majority of Fe (90 at%) is tetrahedrally bonded with O<br />
atoms, i.e. Fe is mainly a glass former. On the contrary, Zn always acts as a glass-former and forms ZnO 4<br />
tetrahedra into the glass matrix, independent of the glass composition.<br />
Acknowledgement: The experimental work was realized with financial support from the EU BESSY-BM.07.1.659 and the<br />
"PYTHAGORAS II" program. One of the authors, F. Pinakidou, acknowledges financial support from the Greek Foundation<br />
of Research Scholarships (IKY).<br />
References<br />
[1] F.G.F. Gibb, Waste Manag., 157 (2000) 27.<br />
[2] M. Pellino et al., Waste Manag., 22 (2002) 945.<br />
[3] F. Pinakidou et al, J. Non-Crystal. Solids, 352 (2006) 2933.<br />
265