xxiii Ïανελληνιο ÏÏ Î½ÎµÎ´Ïιο ÏÏ ÏÎ¹ÎºÎ·Ï ÏÏεÏÎµÎ±Ï ÎºÎ±ÏαÏÏαÏÎ·Ï & εÏιÏÏÎ·Î¼Î·Ï ...
xxiii Ïανελληνιο ÏÏ Î½ÎµÎ´Ïιο ÏÏ ÏÎ¹ÎºÎ·Ï ÏÏεÏÎµÎ±Ï ÎºÎ±ÏαÏÏαÏÎ·Ï & εÏιÏÏÎ·Î¼Î·Ï ...
xxiii Ïανελληνιο ÏÏ Î½ÎµÎ´Ïιο ÏÏ ÏÎ¹ÎºÎ·Ï ÏÏεÏÎµÎ±Ï ÎºÎ±ÏαÏÏαÏÎ·Ï & εÏιÏÏÎ·Î¼Î·Ï ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
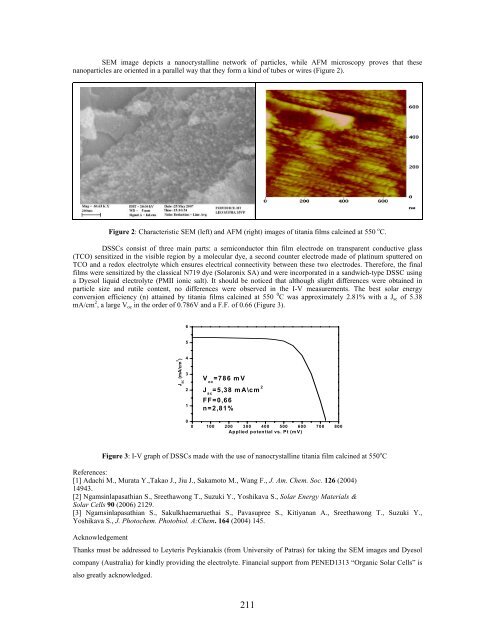
SEM image depicts a nanocrystalline network of particles, while AFM microscopy proves that these<br />
nanoparticles are oriented in a parallel way that they form a kind of tubes or wires (Figure 2).<br />
Figure 2: Characteristic SEM (left) and AFM (right) images of titania films calcined at 550 o C.<br />
DSSCs consist of three main parts: a semiconductor thin film electrode on transparent conductive glass<br />
(TCO) sensitized in the visible region by a molecular dye, a second counter electrode made of platinum sputtered on<br />
TCO and a redox electrolyte which ensures electrical connectivity between these two electrodes. Therefore, the final<br />
films were sensitized by the classical N719 dye (Solaronix SA) and were incorporated in a sandwich-type DSSC using<br />
a Dyesol liquid electrolyte (PMII ionic salt). It should be noticed that although slight differences were obtained in<br />
particle size and rutile content, no differences were observed in the I-V measurements. The best solar energy<br />
conversion efficiency (n) attained by titania films calcined at 550 0 C was approximately 2.81% with a J sc of 5.38<br />
mA/cm 2 , a large V oc in the order of 0.786V and a F.F. of 0.66 (Figure 3).<br />
6<br />
5<br />
J<br />
SC<br />
(mA/cm 2 )<br />
4<br />
3<br />
2<br />
1<br />
V oc<br />
=786 mV<br />
J sc<br />
=5,38 mA\cm 2<br />
FF=0,66<br />
n=2,81%<br />
0<br />
0 100 200 300 400 500 600 700 800<br />
Applied potential vs. Pt (mV)<br />
Figure 3: I-V graph of DSSCs made with the use of nanocrystalline titania film calcined at 550 o C<br />
References:<br />
[1] Adachi M., Murata Y.,Takao J., Jiu J., Sakamoto M., Wang F., J. Am. Chem. Soc. 126 (2004)<br />
14943.<br />
[2] Ngamsinlapasathian S., Sreethawong T., Suzuki Y., Yoshikava S., Solar Energy Materials &<br />
Solar Cells 90 (2006) 2129.<br />
[3] Ngamsinlapasathian S., Sakulkhaemaruethai S., Pavasupree S., Kitiyanan A., Sreethawong T., Suzuki Y.,<br />
Yoshikava S., J. Photochem. Photobiol. A:Chem. 164 (2004) 145.<br />
Acknowledgement<br />
Thanks must be addressed to Leyteris Peykianakis (from University of Patras) for taking the SEM images and Dyesol<br />
company (Australia) for kindly providing the electrolyte. Financial support from PENED1313 “Organic Solar Cells” is<br />
also greatly acknowledged.<br />
211