Download (4Mb) - Etheses - Saurashtra University
Download (4Mb) - Etheses - Saurashtra University
Download (4Mb) - Etheses - Saurashtra University
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Chapter – 3 Preparation of small library…..<br />
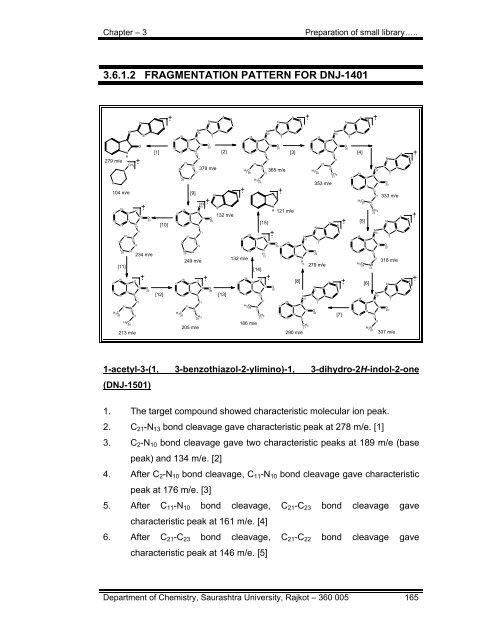
3.6.1.2 FRAGMENTATION PATTERN FOR DNJ-1401<br />
279 m/e<br />
O<br />
N<br />
N<br />
H<br />
1-acetyl-3-(1, 3-benzothiazol-2-ylimino)-1, 3-dihydro-2H-indol-2-one<br />
(DNJ-1501)<br />
N<br />
104 m/e<br />
CH 3<br />
N<br />
O<br />
S<br />
17<br />
16<br />
15<br />
11<br />
18<br />
19<br />
14<br />
12<br />
N<br />
13<br />
21<br />
O<br />
20<br />
27<br />
26<br />
N<br />
22<br />
23<br />
O 25<br />
[11]<br />
24<br />
17<br />
16<br />
15<br />
N<br />
10<br />
11<br />
N<br />
3<br />
2<br />
S<br />
1<br />
9<br />
8<br />
4<br />
7<br />
5<br />
6<br />
17<br />
16<br />
15<br />
N<br />
10<br />
11<br />
N<br />
3<br />
2<br />
S<br />
1<br />
9<br />
8<br />
4<br />
7<br />
5<br />
6<br />
17<br />
16<br />
15<br />
N<br />
10<br />
11<br />
N<br />
3<br />
2<br />
S<br />
1<br />
9<br />
8<br />
4<br />
7<br />
5<br />
6<br />
[1]<br />
18<br />
19<br />
14<br />
12<br />
N<br />
13<br />
O<br />
20<br />
[2]<br />
18<br />
19<br />
14<br />
12<br />
N<br />
13<br />
O<br />
20<br />
[3]<br />
18<br />
19<br />
14<br />
12<br />
N<br />
13<br />
O<br />
20<br />
[4]<br />
21<br />
27<br />
N<br />
22<br />
26<br />
23<br />
17<br />
18<br />
O 25<br />
16<br />
19<br />
24<br />
21<br />
27<br />
N<br />
22<br />
26<br />
23<br />
O 25<br />
NH<br />
10<br />
11<br />
S<br />
15<br />
14<br />
12<br />
N<br />
13<br />
O<br />
20<br />
24<br />
H3C 24<br />
1. The target compound showed characteristic molecular ion peak.<br />
2. C21-N13 bond cleavage gave characteristic peak at 278 m/e. [1]<br />
3. C2-N10 bond cleavage gave two characteristic peaks at 189 m/e (base<br />
peak) and 134 m/e. [2]<br />
4. After C2-N10 bond cleavage, C11-N10 bond cleavage gave characteristic<br />
peak at 176 m/e. [3]<br />
5. After C11-N10 bond cleavage, C21-C23 bond cleavage gave<br />
characteristic peak at 161 m/e. [4]<br />
6. After C21-C23 bond cleavage, C21-C22 bond cleavage gave<br />
characteristic peak at 146 m/e. [5]<br />
Department of Chemistry, <strong>Saurashtra</strong> <strong>University</strong>, Rajkot – 360 005 165<br />
21<br />
27<br />
N<br />
22<br />
H3C 26<br />
23<br />
17<br />
16<br />
15<br />
+. 11<br />
17<br />
16<br />
15<br />
+. 11<br />
17<br />
16<br />
15<br />
11 +.<br />
18<br />
19<br />
14<br />
12<br />
N<br />
13<br />
O<br />
20<br />
[12]<br />
18<br />
19<br />
14<br />
12<br />
N<br />
13<br />
O<br />
20<br />
[13]<br />
18<br />
19<br />
14<br />
12<br />
N<br />
13<br />
O<br />
20<br />
21<br />
21<br />
21<br />
27<br />
H3C 26<br />
N<br />
22<br />
23<br />
27<br />
H3C 26<br />
N<br />
22<br />
CH3 23<br />
H3C 27 N<br />
22<br />
CH3 23<br />
H3C 24<br />
186 m/e<br />
213 m/e<br />
+.<br />
+.<br />
234 m/e<br />
[10]<br />
+.<br />
[9]<br />
249 m/e<br />
205 m/e<br />
378 m/e<br />
+.<br />
N<br />
132 m/e<br />
132 m/e<br />
+. +.<br />
17<br />
18<br />
16<br />
19<br />
CH 3<br />
21<br />
H3C 26<br />
16<br />
2<br />
11<br />
N 5<br />
17 15<br />
10<br />
S<br />
12 O 16<br />
11<br />
1<br />
18 14<br />
20 17 15<br />
19 N<br />
12 O<br />
H 18 14<br />
20<br />
13<br />
19 N<br />
H<br />
13<br />
[14]<br />
[15]<br />
365 m/e<br />
N<br />
H<br />
+.<br />
121 m/e<br />
[8]<br />
N 3<br />
N 3<br />
15<br />
N<br />
10<br />
11<br />
2<br />
S<br />
1<br />
14<br />
12<br />
N<br />
13<br />
O<br />
20<br />
290 m/e<br />
+. +.<br />
27<br />
4<br />
4<br />
5<br />
N 22<br />
353 m/e<br />
279 m/e<br />
9<br />
9<br />
21<br />
CH 3<br />
23<br />
6<br />
6<br />
8<br />
8<br />
7<br />
7<br />
[7]<br />
+.<br />
+.<br />
17<br />
18<br />
17<br />
18<br />
17<br />
18<br />
16<br />
19<br />
H3C 27<br />
16<br />
19<br />
[5]<br />
H3C 27<br />
16<br />
19<br />
N 22<br />
21<br />
CH 3<br />
23<br />
N 3<br />
15<br />
N<br />
10<br />
11<br />
2<br />
S<br />
1<br />
14<br />
12<br />
N<br />
13<br />
O<br />
20<br />
NH<br />
22<br />
21<br />
N 3<br />
15<br />
N<br />
10<br />
11<br />
2<br />
S<br />
1<br />
14<br />
12<br />
N<br />
13<br />
O<br />
20<br />
[6]<br />
H2N 22<br />
21<br />
N 3<br />
15<br />
N<br />
10<br />
11<br />
2<br />
S<br />
1<br />
14<br />
12<br />
N<br />
13<br />
O<br />
20<br />
4<br />
4<br />
4<br />
5<br />
333 m/e<br />
5<br />
318 m/e<br />
307 m/e<br />
5<br />
9<br />
9<br />
9<br />
6<br />
6<br />
6<br />
8<br />
8<br />
8<br />
7<br />
7<br />
7<br />
+.<br />
+.<br />
+.