- Page 1 and 2:
Diagnostik och uppföljning av för
- Page 3:
Diagnostik och uppföljning av för
- Page 6 and 7:
Kvalitetskriterier 75 Urval av stud
- Page 8 and 9:
Child Mania Rating Scale - Parent (
- Page 10 and 11:
Tabeller 294 Referenser 440 3.6 Bed
- Page 12 and 13:
Hur ser läkare och annan vårdpers
- Page 15 and 16:
SBU:s sammanfattning och slutsatser
- Page 17 and 18:
❑❑ ❑❑ Det finns många kuns
- Page 19 and 20:
80 procent utgjordes av egentlig de
- Page 21 and 22:
Diagnostisk tillförlitlighet mäts
- Page 23 and 24:
Faktaruta 3 Studiekvalitet och evid
- Page 25 and 26:
depression (starkt vetenskapligt un
- Page 27 and 28:
Andelen vuxna med förstämningssyn
- Page 29 and 30:
Ett alternativ för barn och ungdom
- Page 31 and 32:
Några formulär för riktad screen
- Page 33 and 34:
Svårighetsgrad av depression och m
- Page 35 and 36:
Vetenskap och praxis överensstämm
- Page 37 and 38:
Bristande kunskap om enskilda formu
- Page 39 and 40:
Slutligen skulle det behövas rutin
- Page 41 and 42:
Tabell 9 fortsättning LCM LEAD MAD
- Page 43:
Tabell 10 fortsättning BDI-II CDSS
- Page 46 and 47:
Vårt uppdrag omfattade en systemat
- Page 48 and 49:
Tabell 1.1 Förstämningssyndromen
- Page 50 and 51:
i barndomen [18]. Avgränsningen mo
- Page 52 and 53:
Utveckling och utvärdering av psyk
- Page 54 and 55:
Bedömning av nyttan av en diagnost
- Page 56 and 57:
Från fyrfältstabellen går det oc
- Page 58 and 59:
Referenser 1. Osby U, Brandt L, Cor
- Page 61 and 62:
2. Metodbeskrivning Syftet med en s
- Page 63 and 64:
Granskarna använde flera olika che
- Page 65 and 66:
Styrka på det vetenskapliga underl
- Page 67:
Referenser 1. Shea BJ, Grimshaw JM,
- Page 70 and 71:
70 diagnostik och uppföljning av f
- Page 72 and 73:
8. Överensstämmer expertskattning
- Page 74 and 75:
Forskare har diskuterat vilken sens
- Page 76 and 77:
QUADAS vidareutvecklades därefter
- Page 78 and 79:
Figur 3.1.1 Flödesschema. För må
- Page 80 and 81:
Referenser 1. Gilbody S, Sheldon T,
- Page 82 and 83:
elevanta originalstudier förutom d
- Page 84 and 85:
Tabell 3.2.1 Resultatsammanställni
- Page 86 and 87:
händertagande, kunde symtombördan
- Page 88 and 89:
Table 3.2.2 Does improved rate of r
- Page 90 and 91:
Referenser 1. Gilbody S, Sheldon T,
- Page 92 and 93:
Introduktion En av våra grundlägg
- Page 94 and 95:
Figur 3.3.2 Forest plot. Spridning
- Page 96 and 97:
Evidensgraderade resultat • Det g
- Page 98 and 99:
eferensstandard som t ex symtomskat
- Page 100 and 101:
Ett genomgående drag i studierna f
- Page 102 and 103:
Table 3.3.3 Are clinical evaluation
- Page 104 and 105:
Table 3.3.3 continued Author Year R
- Page 106 and 107:
Referenser 1. Torrens M, Serrano D,
- Page 108 and 109:
• Det går inte att bedöma sensi
- Page 110 and 111:
semistrukturerad intervju som refer
- Page 112 and 113:
Tabell 3.4.1 Sammanfattning av resu
- Page 114 and 115:
Urval av studier Figur 3.4.1 Flöde
- Page 116 and 117:
psykiatrisk avdelning. Vid utskrivn
- Page 118 and 119:
hypomani hos barn eller ungdomar (o
- Page 120 and 121:
För syndromskalor och DSM-orienter
- Page 122 and 123:
minskade ett steg. Även precisione
- Page 124 and 125:
Evidensgraderat resultat Det går i
- Page 126 and 127:
Figur 3.4.5 Forest plot för sensit
- Page 128 and 129:
Evidensgraderade resultat • Sensi
- Page 130 and 131:
Sammanvägt resultat Sensitiviteten
- Page 132 and 133:
Patient Health Questionnaire (PHQ-9
- Page 134 and 135:
hade 38 procent bipolära syndrom.
- Page 136 and 137:
Tabell 3.4.5 Resultatsammanställni
- Page 138 and 139:
Child Mania Rating Scale - Parent (
- Page 140 and 141:
Slutligen rekryterade Youngstrom oc
- Page 142 and 143:
eferensstandard eftersom det finns
- Page 144 and 145:
sikt av Warnick och medarbetare dä
- Page 146 and 147:
Table 3.4.7 Sensitivity and specifi
- Page 148 and 149:
Table 3.4.8 Sensitivity and specifi
- Page 150 and 151:
Table 3.4.9 Sensitivity and specifi
- Page 152 and 153:
Table 3.4.9 continued Author Year R
- Page 154 and 155:
Table 3.4.10 continued Author Year
- Page 156 and 157:
Table 3.4.12 Child Behavior Checkli
- Page 158 and 159:
Table 3.4.13 P-GBI as screening ins
- Page 160 and 161:
Table 3.4.13 continued Author Year
- Page 162 and 163:
Table 3.4.14 continued Author Year
- Page 164 and 165:
Table 3.4.15 continued Author Year
- Page 166 and 167:
Table 3.4.15 continued Author Year
- Page 168 and 169:
Table 3.4.16b continued Sensitivity
- Page 170 and 171:
Referenser 1. Chambers WJ, Puig-Ant
- Page 172 and 173:
purpose behavior checklist. J Affec
- Page 174 and 175:
58. Ivarsson T, Gillberg C. Depress
- Page 176 and 177:
84. Diler RS, Birmaher B, Axelson D
- Page 178 and 179:
J Am Acad Child Adolesc Psychiatry
- Page 180 and 181:
• I psykiatrisk öppenvård har M
- Page 182 and 183:
i ett brett antal populationer är
- Page 184 and 185:
Bedömning av svårighetsgrad • D
- Page 186 and 187:
Introduktion Syftet med utvärderin
- Page 188 and 189:
Tabell 3.5.1 fortsättning Formulä
- Page 190 and 191:
Urval av studier Av 683 artiklar so
- Page 192 and 193:
Semistrukturerade diagnostiska inte
- Page 194 and 195:
Studien av Ramirez Basco och medarb
- Page 196 and 197:
Tabell 3.5.2 Sammanvägda resultat
- Page 198 and 199:
Schedules for Clinical Assessment i
- Page 200 and 201:
strukturerad intervju som referenss
- Page 202 and 203:
Den sammanvägda sensitiviteten fö
- Page 204 and 205:
• I psykiatrisk öppenvård med m
- Page 206 and 207:
Bedömning av evidensstyrka Det fan
- Page 208 and 209:
Beskrivning av underlaget Det finns
- Page 210 and 211:
Figur 3.5.10 Forest plot för BDI-I
- Page 212 and 213:
Bedömning av evidensstyrka Det fin
- Page 214 and 215:
Tabell 3.5.6 Resultatsammanställni
- Page 216 and 217:
I studien av Leonardoua och medarbe
- Page 218 and 219:
Adouard och medarbetare undersökte
- Page 220 and 221:
Tabell 3.5.7b Resultatsammanställn
- Page 222 and 223:
I den tredje studien intervjuades 2
- Page 224 and 225:
tolkningar. Studierna var välgjord
- Page 226 and 227:
läret skickades ut per post, och e
- Page 228 and 229:
Figur 3.5.14b Forest plot. Sensitiv
- Page 230 and 231:
uppmanas markera på en sexgradig s
- Page 232 and 233:
specificiteten 94 procent för att
- Page 234 and 235:
Den sammanvägda sensitiviteten var
- Page 236 and 237:
nedsättning (det så kallade Genè
- Page 238 and 239:
När standardalgoritmen ersattes me
- Page 240 and 241:
För samtliga fyra jämförelser me
- Page 242 and 243:
på 78 procent (95 % KI, 71 till 84
- Page 244 and 245:
kvalitet. Korrelationen mellan de b
- Page 246 and 247:
version finns som expertskattning (
- Page 248 and 249:
samman över alla frågor (efter om
- Page 250 and 251:
Bech-Rafaelsen Mania Scale (MAS) Be
- Page 252 and 253:
Beskrivning av underlaget Av tio st
- Page 254 and 255:
Figur 3.5.21 Metaanalys: Överensst
- Page 256 and 257:
Den ena studien omfattade 63 patien
- Page 258 and 259:
Av åtta intervjuformulär fanns de
- Page 260 and 261:
En möjlig begränsning i vårt und
- Page 262 and 263:
Studier av samstämmighet har fokus
- Page 264 and 265:
Table 3.5.14 Sensitivity and specif
- Page 266 and 267:
Table 3.5.15 Sensitivity and specif
- Page 268 and 269:
Table 3.5.17 Sensitivity and specif
- Page 270 and 271:
Table 3.5.18 continued Author Year
- Page 272 and 273:
Table 3.5.19 continued Author Year
- Page 274 and 275:
Table 3.5.20 continued Author Year
- Page 276 and 277:
Table 3.5.21 Sensitivity and specif
- Page 278 and 279:
Table 3.5.22 Sensitivity and specif
- Page 280 and 281:
Table 3.5.22 continued Author Year
- Page 282 and 283:
Table 3.5.22 continued Author Year
- Page 284 and 285: Table 3.5.22 continued Author Year
- Page 286 and 287: Table 3.5.22 continued Author Year
- Page 288 and 289: Table 3.5.22 continued Author Year
- Page 290 and 291: Table 3.5.22 continued Author Year
- Page 292 and 293: Table 3.5.22 continued Author Year
- Page 294 and 295: Table 3.5.23 Sensitivity and specif
- Page 296 and 297: Table 3.5.23 continued Author Year
- Page 298 and 299: Table 3.5.23 continued Author Year
- Page 300 and 301: Table 3.5.23 continued Author Year
- Page 302 and 303: Table 3.5.24 continued Author Year
- Page 304 and 305: Table 3.5.24 continued Author Year
- Page 306 and 307: Table 3.5.24 continued Author Year
- Page 308 and 309: Table 3.5.24 continued Author Year
- Page 310 and 311: Table 3.5.26 Sensitivity and specif
- Page 312 and 313: Table 3.5.27 Sensitivity and specif
- Page 314 and 315: Table 3.5.28 Sensitivity and specif
- Page 316 and 317: Table 3.5.28 continued Author Year
- Page 318 and 319: Table 3.5.28 continued Author Year
- Page 320 and 321: Table 3.5.29 continued Author Year
- Page 322 and 323: Table 3.5.30 Which diagnoses are as
- Page 324 and 325: Table 3.5.32 Calgary Depression Sca
- Page 326 and 327: Table 3.5.34 MADRS for assessment o
- Page 328 and 329: Table 3.5.36 MAS for assessment of
- Page 330 and 331: Table 3.5.38 LCM-p for assessment o
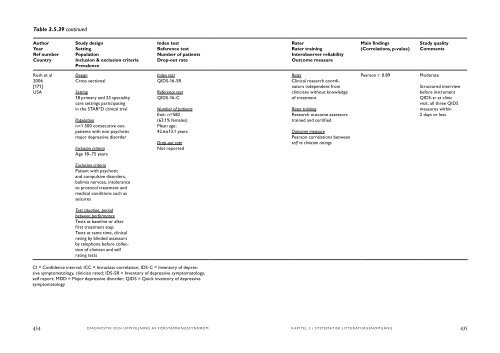
- Page 332 and 333: Table 3.5.39 continued Author Year
- Page 336 and 337: Table 3.5.41 Correlation between cl
- Page 338 and 339: sources of variance of the composit
- Page 340 and 341: 43. Addington D, Addington J, Matic
- Page 342 and 343: and prevalence of postnatal depress
- Page 344 and 345: 98. WHO. The ICD-10 Classification
- Page 346 and 347: M, et al. The mood disorder questio
- Page 348 and 349: eference to DSM-IV and ICD-10. 2nd
- Page 350 and 351: and postpartum women. Acta Psychiat
- Page 352 and 353: 3.6 Bedömningsformulär för äldr
- Page 354 and 355: Depression hos äldre kan ha hetero
- Page 356 and 357: Tabell 3.6.1 Sammanfattning av resu
- Page 358 and 359: Det vetenskapliga underlaget bestod
- Page 360 and 361: Geriatric Depression Scale (GDS-15)
- Page 362 and 363: Figur 3.6.4 Forest plot för sensit
- Page 364 and 365: Hospital Anxiety and Depression Sca
- Page 366 and 367: Tabell 3.6.4 Resultatsammanställni
- Page 368 and 369: Tabell 3.6.5 Resultatsammanställni
- Page 370 and 371: Center for Epidemiologic Studies De
- Page 372 and 373: person och något mindre tillförli
- Page 374 and 375: gångna studierna. Behovet av att m
- Page 376 and 377: KAPITEL 3 • Systematisk litteratu
- Page 378 and 379: Table 3.6.6 continued Author Year R
- Page 380 and 381: Table 3.6.7 continued Author Year R
- Page 382 and 383: Table 3.6.7 continued Author Year R
- Page 384 and 385:
Table 3.6.8 Hospital Anxiety and De
- Page 386 and 387:
Table 3.6.9 PHQ-9 for screening of
- Page 388 and 389:
Table 3.6.9 continued Author Year R
- Page 390 and 391:
Table 3.6.11 BDI-II for screening o
- Page 392 and 393:
Table 3.6.13 SIDI for screening of
- Page 394 and 395:
and cognitive decline in nondemente
- Page 396:
a systematic review. Acta Psychiatr
- Page 399 and 400:
Table 3.7.1 Health economy outcome.
- Page 401 and 402:
4. Inventering Det finns mängder a
- Page 403 and 404:
KAPITEL 4 • inventering 523
- Page 405 and 406:
Tabell 4.1 fortsättning Formulär
- Page 407 and 408:
Tabell 4.2 fortsättning Formulär
- Page 409 and 410:
Tabell 4.3 Formulär för riktad sc
- Page 411 and 412:
Referenser 1. Nezu AM, Ronan GF, Me
- Page 413 and 414:
sion of PRIME-MD: the PHQ primary c
- Page 416 and 417:
5. Klinisk betydelse av prestanda h
- Page 418 and 419:
Diagnostik av depression med PHQ-9
- Page 420 and 421:
Tabell 5.6 Sammanställning för MD
- Page 422 and 423:
6. Etiska och sociala aspekter Dett
- Page 424 and 425:
Vilka är de etiska aspekterna på
- Page 426 and 427:
Vilka är de etiska aspekterna på
- Page 428 and 429:
Ett konkret exempel på etiskt prob
- Page 430:
gram is missing]. Lakartidningen 20
- Page 433 and 434:
Vetenskapligt underlag Vårt underl
- Page 435 and 436:
Figur 7.1 Användning av strukturer
- Page 437 and 438:
götland hade vårdprogram för bar
- Page 439 and 440:
ter gick det att genomföra en inte
- Page 441 and 442:
Ingen av deltagarna i fokusgruppern
- Page 443 and 444:
Referenser 1. Demyttenaere K, De Fr
- Page 446 and 447:
8. Kunskapsluckor Vår granskning v
- Page 448 and 449:
9. Konsekvenser av rapportens resul
- Page 450 and 451:
Idag finns det ingen lättillgängl
- Page 452:
Referenser 1. Socialstyrelsen. Nati
- Page 455 and 456:
Depression Degenerativ hjärnsjukdo
- Page 457 and 458:
Inklusionskriterium Interbedömarre
- Page 459 and 460:
Pilotstudie Positivt/ negativt pred
- Page 461 and 462:
Screening Semistrukturerad intervju
- Page 463 and 464:
Förkortningslista - Bedömningsfor
- Page 465 and 466:
DICA-IV DIS DISC-IV DSRS EPDS GDS G
- Page 467 and 468:
MDQ-A MINI P-GBI PHQ-2 PHQ-9 PRIME-
- Page 470 and 471:
11. Projektgrupp, externa granskare
- Page 472 and 473:
Externa vetenskapliga granskare Mal
- Page 474 and 475:
Metoder för tidig fosterdiagnostik
- Page 476:
Rapporter på engelska (2001-2012)