TABLE OF CONTENTS - Lindbergh School District
TABLE OF CONTENTS - Lindbergh School District
TABLE OF CONTENTS - Lindbergh School District
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
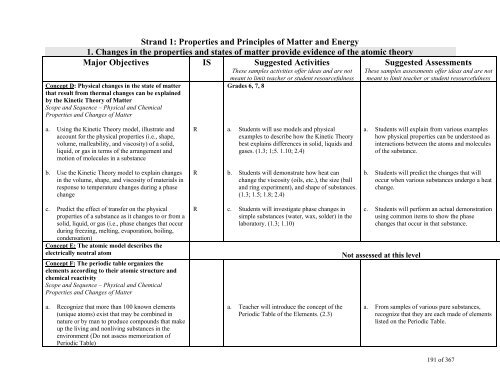
Strand 1: Properties and Principles of Matter and Energy<br />
1. Changes in the properties and states of matter provide evidence of the atomic theory<br />
Major Objectives IS Suggested Activities<br />
These samples activities offer ideas and are not<br />
Concept D: Physical changes in the state of matter<br />
that result from thermal changes can be explained<br />
by the Kinetic Theory of Matter<br />
Scope and Sequence – Physical and Chemical<br />
Properties and Changes of Matter<br />
meant to limit teacher or student resourcefulness<br />
Grades 6, 7, 8<br />
Suggested Assessments<br />
These samples assessments offer ideas and are not<br />
meant to limit teacher or student resourcefulness<br />
a. Using the Kinetic Theory model, illustrate and<br />
account for the physical properties (i.e., shape,<br />
volume, malleability, and viscosity) of a solid,<br />
liquid, or gas in terms of the arrangement and<br />
motion of molecules in a substance<br />
R<br />
a. Students will use models and physical<br />
examples to describe how the Kinetic Theory<br />
best explains differences in solid, liquids and<br />
gases. (1.3; 1;5. 1.10; 2.4)<br />
a. Students will explain from various examples<br />
how physical properties can be understood as<br />
interactions between the atoms and molecules<br />
of the substance.<br />
b. Use the Kinetic Theory model to explain changes<br />
in the volume, shape, and viscosity of materials in<br />
response to temperature changes during a phase<br />
change<br />
R<br />
b. Students will demonstrate how heat can<br />
change the viscosity (oils, etc.), the size (ball<br />
and ring experiment), and shape of substances.<br />
(1.3; 1.5; 1.8; 2.4)<br />
b. Students will predict the changes that will<br />
occur when various substances undergo a heat<br />
change.<br />
c. Predict the effect of transfer on the physical<br />
properties of a substance as it changes to or from a<br />
solid, liquid, or gas (i.e., phase changes that occur<br />
during freezing, melting, evaporation, boiling,<br />
condensation)<br />
Concept E: The atomic model describes the<br />
electrically neutral atom<br />
Concept F: The periodic table organizes the<br />
elements according to their atomic structure and<br />
chemical reactivity<br />
Scope and Sequence – Physical and Chemical<br />
Properties and Changes of Matter<br />
R<br />
c. Students will investigate phase changes in<br />
simple substances (water, wax, solder) in the<br />
laboratory. (1.3; 1.10)<br />
c. Students will perform an actual demonstration<br />
using common items to show the phase<br />
changes that occur in that substance.<br />
Not assessed at this level<br />
a. Recognize that more than 100 known elements<br />
(unique atoms) exist that may be combined in<br />
nature or by man to produce compounds that make<br />
up the living and nonliving substances in the<br />
environment (Do not assess memorization of<br />
Periodic Table)<br />
a. Teacher will introduce the concept of the<br />
Periodic Table of the Elements. (2.3)<br />
a. From samples of various pure substances,<br />
recognize that they are each made of elements<br />
listed on the Periodic Table.<br />
191 of 367