Ce document est le fruit d'un long travail approuvé par le jury de ...
Ce document est le fruit d'un long travail approuvé par le jury de ...
Ce document est le fruit d'un long travail approuvé par le jury de ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
2 The C3-symmetrical tris-ACE-6,6’-bipyridyl--CyD tripod 9<br />
14 was synthesized from 6 A ,6 C ,6 E -triazido-6 A ,6 C ,6 E -tri<strong>de</strong>oxy-<br />
6 B ,6 D ,6 F -tri-O-acetyl-hexakis-2,3-di-O-acetyl-cyclomaltohexaose<br />
8 8 (scheme 1) by the tan<strong>de</strong>m Staudinger-Aza-Wittig (SAW) key<br />
reaction in 50 % yield 9 (Scheme 1). The monoaminomethyl-6methyl-6’-2,2’-bipyridin<br />
4 was obtained in three steps from 6,6’dimethyl-2,2’-bipyridin<br />
1. 10 Compounds 6-8 were pre<strong>par</strong>ed by<br />
known methods. 11 All compounds 2-9 were characterized by<br />
current spectroscopic methods. The spectroscopic data are in<br />
agreement with the assigned structures. (see experimental section<br />
). 12 As expected, the IR spectrum of 9 exhibited characteristic<br />
frequencies of the carbonyl functions: urea CO-NH at 1640 cm -1 ;<br />
<strong>est</strong>er CO-O at 1750cm -1 (acetates) and aromatic doub<strong>le</strong> bonds of<br />
bipyridyl groups at 1560 cm -1 . The 13 C-NMR spectrum in<br />
solution (DMSO) indicate that the ligand have a C3-symmetry.<br />
The signals corresponding to the NH-CO-NH carbonyl carbons at<br />
158.1 ppm, the CO-O carbonyl carbons at 171.4 to 162.9 ppm<br />
and the signals corresponding to the bipyridyl carbons from <br />
138.1 to 118.5 ppm were <strong>de</strong>tected.<br />
Metal ion comp<strong>le</strong>xation<br />
The e<strong>le</strong>ctronic spectrum of the free tripod 9 recor<strong>de</strong>d in<br />
MeOH shows two maxima at max = 244 nm (14700 mol -1 .dm 3<br />
.cm -1 ) and max = 290 nm (32000 mol -1 .dm 3 .cm -1 ). These<br />
absorptions correspond to the -* transitions of bipyridyls and<br />
to the n-* urea and carboxylate carbonyl doub<strong>le</strong> bonds.<br />
Figure 1. Schematic representation of the -CyD tripod 9 and its potent<br />
comp<strong>le</strong>xation sites.<br />
Figure 2. Postulated folding pathway for the metal ion-assisted selfassembly<br />
of Cu II and Fe III /Cu II <strong>le</strong>ft-han<strong>de</strong>d (M) trip<strong>le</strong>-helices of 9.<br />
The extinction value calculated from the mo<strong>le</strong>cular extinction<br />
coefficient in 9 is c.a. 10700 mol -1 .dm 3 .cm -1 per bipyridine unit ;<br />
this value is within the range found in the literature. 15 As<br />
illustrated in Figure 1, the tripod 9 represents the first term of a<br />
new set of heterotritopic mo<strong>le</strong>cular receptor having two potent<br />
metal coordination sites: one formed by the bipyridyl units and<br />
the second by ureas and /or carboxylates, the third site is formed<br />
by the CyD torus cavity, <strong>de</strong>dicated to the formation of host-gu<strong>est</strong><br />
comp<strong>le</strong>xes via inclusion of small organic hydrophobic mo<strong>le</strong>cu<strong>le</strong>s.<br />
Tetrahedron Letters<br />
The titrations of 9 with “hard” to “bor<strong>de</strong>rline” (HSAB theory)<br />
cations were monitored by Uv-Vis spectroscopy. As expected<br />
and based on results previously <strong>est</strong>ablished in our research group,<br />
“bor<strong>de</strong>rline” cations as Cu II and Ni II were comp<strong>le</strong>xed at the<br />
bipyridyls whi<strong>le</strong> “hard” cations as Fe III or Ru III were coordinated<br />
to the carbony<strong>le</strong> urea/<strong>est</strong>er functions (Figure 2.) according to the<br />
HSAB theory. The titration (Figure 3.) and the ligand speciation<br />
curves (Figure 4.) showed examp<strong>le</strong>s of mononuc<strong>le</strong>ar podandate<br />
formation of [1:1] stoichiometry [9/Cu II ] and [1:1:1] dinuc<strong>le</strong>ar<br />
podandate [9/Fe III /Cu II ].<br />
(A) (dinuc<strong>le</strong>ar comp<strong>le</strong>x)<br />
(B)<br />
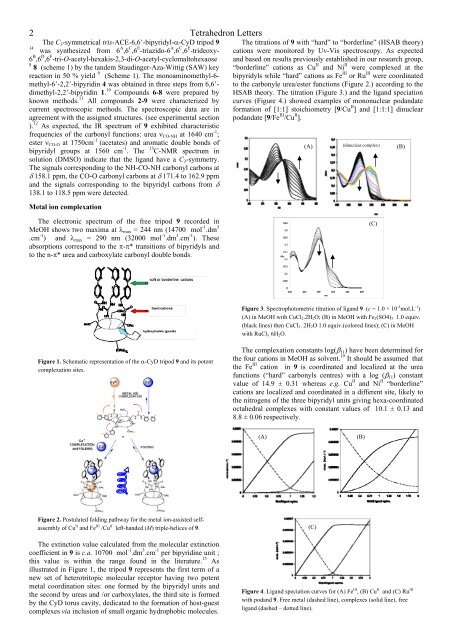
Figure 3. Spectrophotometric titration of ligand 9 (c = 1.0 × 10 -5 mol.L -1 )<br />
(A) in MeOH with CuCl2.2H2O; (B) in MeOH with Fe2(SO4)3 1.0 equiv.<br />
(black lines) then CuCl2. 2H2O 1.0 equiv.(colored lines); (C) in MeOH<br />
with RuCl3 .6H2O.<br />
The comp<strong>le</strong>xation constants log( 11) have been <strong>de</strong>termined for<br />
the four cations in MeOH as solvent. 19 It should be assumed that<br />
the Fe III cation in 9 is coordinated and localized at the urea<br />
functions (“hard” carbonyls centres) with a log ( 11) constant<br />
value of 14.9 ± 0.31 whereas e.g. Cu II and Ni II “bor<strong>de</strong>rline”<br />
cations are localized and coordinated in a different site, likely to<br />
the nitrogens of the three bipyridyl units giving hexa-coordinated<br />
octahedral comp<strong>le</strong>xes with constant values of 10.1 ± 0.13 and<br />
8.8 ± 0.06 respectively.<br />
(A) (B)<br />
(C)<br />
Figure 4. Ligand speciation curves for (A) Fe III , (B) Cu II and (C) Ru III<br />
with podand 9. Free metal (dashed line), comp<strong>le</strong>xes (solid line), free<br />
ligand (dashed – dotted line).<br />
(C)