Ce document est le fruit d'un long travail approuvé par le jury de ...
Ce document est le fruit d'un long travail approuvé par le jury de ...
Ce document est le fruit d'un long travail approuvé par le jury de ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Formation of a trip<strong>le</strong> helix by coordination with metal cations<br />
was ascertained by Circular Dichroïsm. As expected but<br />
differently of 5,5’analogues 5 , coordination of ureido- 6,6’bipyridyls<br />
arms with e.g. Cu II cation spontaneously generates a<br />
strong exciton coupling-type positive Cotton effect ( = + 33<br />
mol -1 .cm -1 ) between the bipyridine units (i.e. a negative band at<br />
290nm, a positive band at 315 nm and an isodichroic point at<br />
approximatively 302 nm) proving a high helicity induction<br />
(Figure 5.).<br />
(A)<br />
(B)<br />
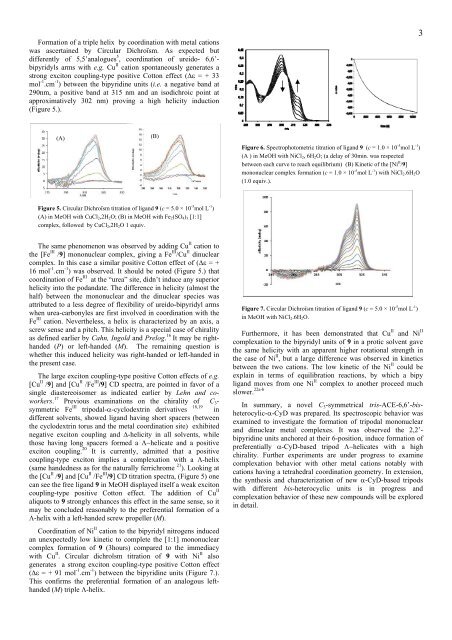
Figure 5. Circular Dichroïsm titration of ligand 9 (c = 5.0 × 10 -5 mol L -1 )<br />
(A) in MeOH with CuCl2,2H2O; (B) in MeOH with Fe2(SO4)3 [1:1]<br />
comp<strong>le</strong>x, followed by CuCl2,2H2O 1 equiv.<br />
The same phenomenon was observed by adding Cu II cation to<br />
the [Fe III /9] mononuc<strong>le</strong>ar comp<strong>le</strong>x, giving a Fe III /Cu II dinuc<strong>le</strong>ar<br />
comp<strong>le</strong>x. In this case a similar positive Cotton effect of ( = +<br />
16 mol -1 .cm -1 ) was observed. It should be noted (Figure 5.) that<br />
coordination of Fe III at the “urea” site, didn’t induce any superior<br />
helicity into the podandate. The difference in helicity (almost the<br />
half) between the mononuc<strong>le</strong>ar and the dinuc<strong>le</strong>ar species was<br />
attributed to a <strong>le</strong>ss <strong>de</strong>gree of f<strong>le</strong>xibility of ureido-bipyridyl arms<br />
when urea-carbony<strong>le</strong>s are first involved in coordination with the<br />
Fe III cation. Neverthe<strong>le</strong>ss, a helix is characterized by an axis, a<br />
screw sense and a pitch. This helicity is a special case of chirality<br />
as <strong>de</strong>fined earlier by Cahn, Ingold and Prelog. 16 It may be righthan<strong>de</strong>d<br />
(P) or <strong>le</strong>ft-han<strong>de</strong>d (M). The remaining qu<strong>est</strong>ion is<br />
whether this induced helicity was right-han<strong>de</strong>d or <strong>le</strong>ft-han<strong>de</strong>d in<br />
the present case.<br />
The large exciton coupling-type positive Cotton effects of e.g.<br />
[Cu II /9] and [Cu II /Fe III /9] CD spectra, are pointed in favor of a<br />
sing<strong>le</strong> diastereoisomer as indicated earlier by Lehn and coworkers.<br />
17 Previous examinations on the chirality of C 3symmetric<br />
Fe III tripodal--cyclo<strong>de</strong>xtrin <strong>de</strong>rivatives 18,19 in<br />
different solvents, showed ligand having short spacers (between<br />
the cyclo<strong>de</strong>xtrin torus and the metal coordination site) exhibited<br />
negative exciton coupling and -helicity in all solvents, whi<strong>le</strong><br />
those having <strong>long</strong> spacers formed a helicate and a positive<br />
exciton coupling. 20 It is currently, admitted that a positive<br />
coupling-type exciton implies a comp<strong>le</strong>xation with a -helix<br />
(same han<strong>de</strong>dness as for the naturally ferrichrome 21 ). Looking at<br />
the [Cu II /9] and [Cu II /Fe III /9] CD titration spectra, (Figure 5) one<br />
can see the free ligand 9 in MeOH displayed itself a weak exciton<br />
coupling-type positive Cotton effect. The addition of Cu II<br />
aliquots to 9 strongly enhances this effect in the same sense, so it<br />
may be conclu<strong>de</strong>d reasonably to the preferential formation of a<br />
-helix with a <strong>le</strong>ft-han<strong>de</strong>d screw propel<strong>le</strong>r (M).<br />
Coordination of Ni II cation to the bipyridyl nitrogens induced<br />
an unexpectedly low kinetic to comp<strong>le</strong>te the [1:1] mononuc<strong>le</strong>ar<br />
comp<strong>le</strong>x formation of 9 (3hours) com<strong>par</strong>ed to the immediacy<br />
with Cu II . Circular dichroïsm titration of 9 with Ni II also<br />
generates a strong exciton coupling-type positive Cotton effect<br />
( = + 91 mol -1 .cm -1 ) between the bipyridine units (Figure 7.).<br />
This confirms the preferential formation of an analogous <strong>le</strong>fthan<strong>de</strong>d<br />
(M) trip<strong>le</strong> -helix.<br />
Figure 6. Spectrophotometric titration of ligand 9 (c = 1.0 × 10 -5 mol L -1 )<br />
(A ) in MeOH with NiCl2, 6H2O; (a <strong>de</strong>lay of 30min. was respected<br />
between each curve to reach equilibrium) (B) Kinetic of the [Ni II /9]<br />
mononuc<strong>le</strong>ar comp<strong>le</strong>x formation (c = 1.0 × 10 -5 mol L -1 ) with NiCl2.6H2O<br />
(1.0 equiv.).<br />
Figure 7. Circular Dichroïsm titration of ligand 9 (c = 5.0 × 10 -5 mol L -1 )<br />
in MeOH with NiCl2.6H2O.<br />
Furthermore, it has been <strong>de</strong>monstrated that Cu II and Ni II<br />
comp<strong>le</strong>xation to the bipyridyl units of 9 in a protic solvent gave<br />
the same helicity with an ap<strong>par</strong>ent higher rotational strength in<br />
the case of Ni II , but a large difference was observed in kinetics<br />
between the two cations. The low kinetic of the Ni II could be<br />
explain in terms of equilibration reactions, by which a bipy<br />
ligand moves from one Ni II comp<strong>le</strong>x to another proceed much<br />
slower. 22a-b<br />
In summary, a novel C 3-symmetrical tris-ACE-6,6’-bisheterocylic--CyD<br />
was pre<strong>par</strong>ed. Its spectroscopic behavior was<br />
examined to inv<strong>est</strong>igate the formation of tripodal mononuc<strong>le</strong>ar<br />
and dinuc<strong>le</strong>ar metal comp<strong>le</strong>xes. It was observed the 2,2’bipyridine<br />
units anchored at their 6-position, induce formation of<br />
preferentially -CyD-based tripod helicates with a high<br />
chirality. Further experiments are un<strong>de</strong>r progress to examine<br />
comp<strong>le</strong>xation behavior with other metal cations notably with<br />
cations having a tetrahedral coordination geometry. In extension,<br />
the synthesis and characterization of new -CyD-based tripods<br />
with different bis-heterocyclic units is in progress and<br />
comp<strong>le</strong>xation behavior of these new compounds will be explored<br />
in <strong>de</strong>tail.<br />
3