Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
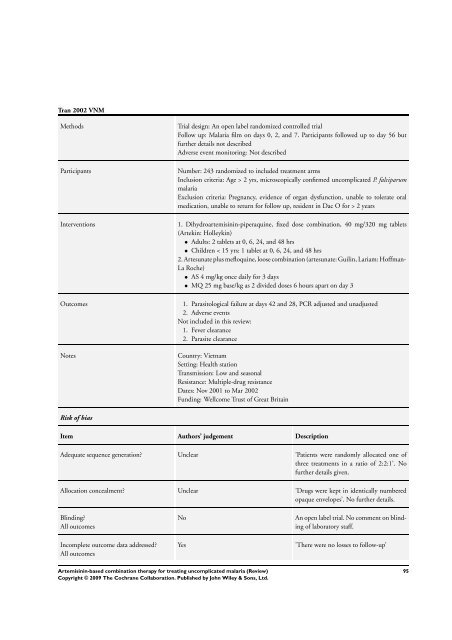
Tran 2002 VNM<br />
Methods Trial design: An open label randomized controlled trial<br />
Follow up: Malaria film on days 0, 2, and 7. Participants followed up to day 56 but<br />
further details not described<br />
Adverse event monitoring: Not described<br />
Participants Number: 243 randomized to included treatment arms<br />
Inclusion criteria: Age > 2 yrs, microscopically confirmed uncomplicated P. falciparum<br />
malaria<br />
Exclusion criteria: Pregnancy, evidence of organ dysfunction, unable to tolerate oral<br />
medication, unable to return <strong>for</strong> follow up, resident in Dac O <strong>for</strong> > 2 years<br />
Interventions 1. Dihydroartemisinin-piperaquine, fixed dose <strong>combination</strong>, 40 mg/320 mg tablets<br />
(Artekin: Holleykin)<br />
• Adults: 2 tablets at 0, 6, 24, and 48 hrs<br />
• Children < 15 yrs: 1 tablet at 0, 6, 24, and 48 hrs<br />
2. Artesunate plus mefloquine, loose <strong>combination</strong> (artesunate: Guilin, Lariam: Hoffman-<br />
La Roche)<br />
• AS 4 mg/kg once daily <strong>for</strong> 3 days<br />
• MQ 25 mg base/kg as 2 divided doses 6 hours apart on day 3<br />
Outcomes 1. Parasitological failure at days 42 and 28, PCR adjusted and unadjusted<br />
2. Adverse events<br />
Not included in this review:<br />
1. Fever clearance<br />
2. Parasite clearance<br />
Notes Country: Vietnam<br />
Setting: Health station<br />
Transmission: Low and seasonal<br />
Resistance: Multiple-drug resistance<br />
Dates: Nov 2001 to Mar 2002<br />
Funding: Wellcome Trust of Great Britain<br />
Risk of bias<br />
Item Authors’ judgement Description<br />
Adequate sequence generation? Unclear ’Patients were randomly allocated one of<br />
three treatments in a ratio of 2:2:1’. No<br />
further details given.<br />
Allocation concealment? Unclear ’Drugs were kept in identically numbered<br />
opaque envelopes’. No further details.<br />
Blinding?<br />
All outcomes<br />
Incomplete outcome data addressed?<br />
All outcomes<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
No An open label trial. No comment on blinding<br />
of laboratory staff.<br />
Yes ’<strong>The</strong>re were no losses to follow-up’<br />
95