Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
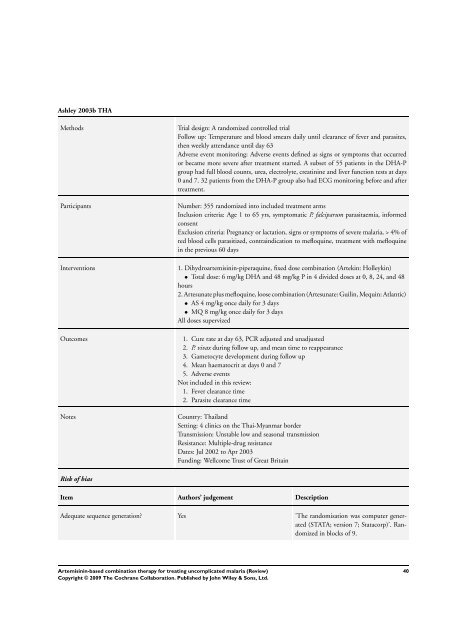
Ashley 2003b THA<br />
Methods Trial design: A randomized controlled trial<br />
Follow up: Temperature and blood smears daily until clearance of fever and parasites,<br />
then weekly attendance until day 63<br />
Adverse event monitoring: Adverse events defined as signs or symptoms that occurred<br />
or became more severe after treatment started. A subset of 55 patients in the DHA-P<br />
group had full blood counts, urea, electrolyte, creatinine and liver function tests at days<br />
0 and 7. 32 patients from the DHA-P group also had ECG monitoring be<strong>for</strong>e and after<br />
treatment.<br />
Participants Number: 355 randomized into included treatment arms<br />
Inclusion criteria: Age 1 to 65 yrs, symptomatic P. falciparum parasitaemia, in<strong>for</strong>med<br />
consent<br />
Exclusion criteria: Pregnancy or lactation, signs or symptoms of severe malaria, > 4% of<br />
red blood cells parasitized, contraindication to mefloquine, treatment with mefloquine<br />
in the previous 60 days<br />
Interventions 1. Dihydroartemisinin-piperaquine, fixed dose <strong>combination</strong> (Artekin: Holleykin)<br />
• Total dose: 6 mg/kg DHA and 48 mg/kg P in 4 divided doses at 0, 8, 24, and 48<br />
hours<br />
2. Artesunate plus mefloquine, loose <strong>combination</strong> (Artesunate: Guilin, Mequin: Atlantic)<br />
• AS 4 mg/kg once daily <strong>for</strong> 3 days<br />
• MQ 8 mg/kg once daily <strong>for</strong> 3 days<br />
All doses supervized<br />
Outcomes 1. Cure rate at day 63, PCR adjusted and unadjusted<br />
2. P. vivax during follow up, and mean time to reappearance<br />
3. Gametocyte development during follow up<br />
4. Mean haematocrit at days 0 and 7<br />
5. Adverse events<br />
Not included in this review:<br />
1. Fever clearance time<br />
2. Parasite clearance time<br />
Notes Country: Thailand<br />
Setting: 4 clinics on the Thai-Myanmar border<br />
Transmission: Unstable low and seasonal transmission<br />
Resistance: Multiple-drug resistance<br />
Dates: Jul 2002 to Apr 2003<br />
Funding: Wellcome Trust of Great Britain<br />
Risk of bias<br />
Item Authors’ judgement Description<br />
Adequate sequence generation? Yes ’<strong>The</strong> randomisation was computer generated<br />
(STATA; version 7; Statacorp)’. Randomized<br />
in blocks of 9.<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
40