Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
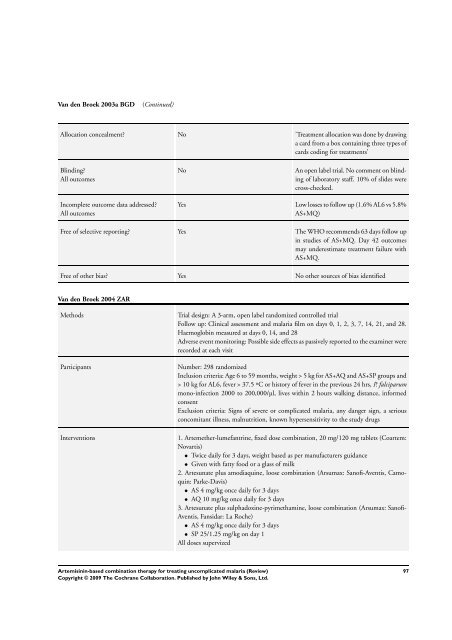
Van den Broek 2003a BGD (Continued)<br />
Allocation concealment? No ’Treatment allocation was done by drawing<br />
a card from a box containing three types of<br />
cards coding <strong>for</strong> treatments’<br />
Blinding?<br />
All outcomes<br />
Incomplete outcome data addressed?<br />
All outcomes<br />
No An open label trial. No comment on blinding<br />
of laboratory staff. 10% of slides were<br />
cross-checked.<br />
Yes Low losses to follow up (1.6% AL6 vs 5.8%<br />
AS+MQ)<br />
Free of selective reporting? Yes <strong>The</strong> WHO recommends 63 days follow up<br />
in studies of AS+MQ. Day 42 outcomes<br />
may underestimate treatment failure with<br />
AS+MQ.<br />
Free of other bias? Yes No other sources of bias identified<br />
Van den Broek 2004 ZAR<br />
Methods Trial design: A 3-arm, open label randomized controlled trial<br />
Follow up: Clinical assessment and malaria film on days 0, 1, 2, 3, 7, 14, 21, and 28.<br />
Haemoglobin measured at days 0, 14, and 28<br />
Adverse event monitoring: Possible side effects as passively reported to the examiner were<br />
recorded at each visit<br />
Participants Number: 298 randomized<br />
Inclusion criteria: Age 6 to 59 months, weight > 5 kg <strong>for</strong> AS+AQ and AS+SP groups and<br />
> 10 kg <strong>for</strong> AL6, fever > 37.5 ºC or history of fever in the previous 24 hrs, P. falciparum<br />
mono-infection 2000 to 200,000/µl, lives within 2 hours walking distance, in<strong>for</strong>med<br />
consent<br />
Exclusion criteria: Signs of severe or complicated malaria, any danger sign, a serious<br />
concomitant illness, malnutrition, known hypersensitivity to the study drugs<br />
Interventions 1. Artemether-lumefantrine, fixed dose <strong>combination</strong>, 20 mg/120 mg tablets (Coartem:<br />
Novartis)<br />
• Twice daily <strong>for</strong> 3 days, weight <strong>based</strong> as per manufacturers guidance<br />
• Given with fatty food or a glass of milk<br />
2. Artesunate plus amodiaquine, loose <strong>combination</strong> (Arsumax: Sanofi-Aventis, Camoquin:<br />
Parke-Davis)<br />
• AS 4 mg/kg once daily <strong>for</strong> 3 days<br />
• AQ 10 mg/kg once daily <strong>for</strong> 3 days<br />
3. Artesunate plus sulphadoxine-pyrimethamine, loose <strong>combination</strong> (Arsumax: Sanofi-<br />
Aventis, Fansidar: La Roche)<br />
• AS 4 mg/kg once daily <strong>for</strong> 3 days<br />
• SP 25/1.25 mg/kg on day 1<br />
All doses supervized<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
97