Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
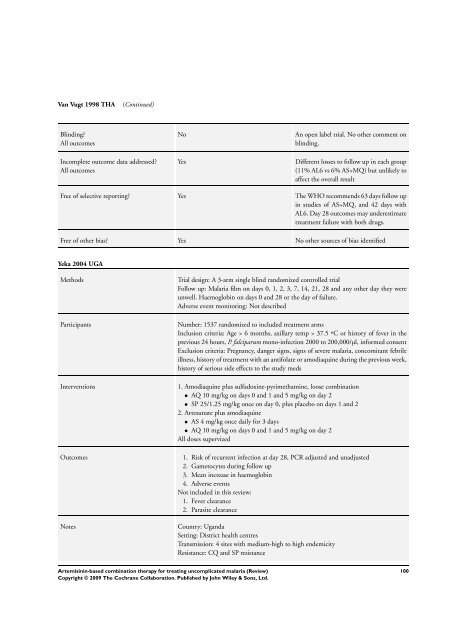
Van Vugt 1998 THA (Continued)<br />
Blinding?<br />
All outcomes<br />
Incomplete outcome data addressed?<br />
All outcomes<br />
No An open label trial. No other comment on<br />
blinding.<br />
Yes Different losses to follow up in each group<br />
(11% AL6 vs 6% AS+MQ) but unlikely to<br />
affect the overall result<br />
Free of selective reporting? Yes <strong>The</strong> WHO recommends 63 days follow up<br />
in studies of AS+MQ, and 42 days with<br />
AL6. Day 28 outcomes may underestimate<br />
treatment failure with both drugs.<br />
Free of other bias? Yes No other sources of bias identified<br />
Yeka 2004 UGA<br />
Methods Trial design: A 3-arm single blind randomized controlled trial<br />
Follow up: Malaria film on days 0, 1, 2, 3, 7, 14, 21, 28 and any other day they were<br />
unwell. Haemoglobin on days 0 and 28 or the day of failure.<br />
Adverse event monitoring: Not described<br />
Participants Number: 1537 randomized to included treatment arms<br />
Inclusion criteria: Age > 6 months, axillary temp > 37.5 ºC or history of fever in the<br />
previous 24 hours, P. falciparum mono-infection 2000 to 200,000/µl, in<strong>for</strong>med consent<br />
Exclusion criteria: Pregnancy, danger signs, signs of severe malaria, concomitant febrile<br />
illness, history of treatment with an antifolate or amodiaquine during the previous week,<br />
history of serious side effects to the study meds<br />
Interventions 1. Amodiaquine plus sulfadoxine-pyrimethamine, loose <strong>combination</strong><br />
• AQ 10 mg/kg on days 0 and 1 and 5 mg/kg on day 2<br />
• SP 25/1.25 mg/kg once on day 0, plus placebo on days 1 and 2<br />
2. Artesunate plus amodiaquine<br />
• AS 4 mg/kg once daily <strong>for</strong> 3 days<br />
• AQ 10 mg/kg on days 0 and 1 and 5 mg/kg on day 2<br />
All doses supervized<br />
Outcomes 1. Risk of recurrent infection at day 28, PCR adjusted and unadjusted<br />
2. Gametocytes during follow up<br />
3. Mean increase in haemoglobin<br />
4. Adverse events<br />
Not included in this review:<br />
1. Fever clearance<br />
2. Parasite clearance<br />
Notes Country: Uganda<br />
Setting: District health centres<br />
Transmission: 4 sites with medium-high to high endemicity<br />
Resistance: CQ and SP resistance<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
100