Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
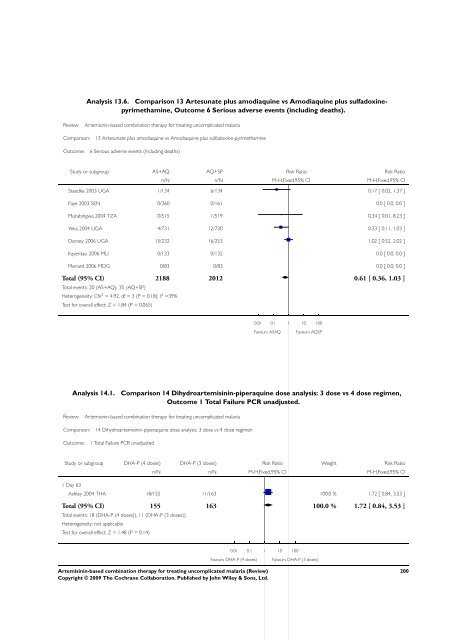
Analysis 13.6. Comparison 13 Artesunate plus amodiaquine vs Amodiaquine plus sulfadoxinepyrimethamine,<br />
Outcome 6 Serious adverse events (including deaths).<br />
Review: <strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria<br />
Comparison: 13 Artesunate plus amodiaquine vs Amodiaquine plus sulfadoxine-pyrimethamine<br />
Outcome: 6 Serious adverse events (including deaths)<br />
Study or subgroup AS+AQ AQ+SP Risk Ratio Risk Ratio<br />
n/N n/N M-H,Fixed,95% CI M-H,Fixed,95% CI<br />
Staedke 2003 UGA 1/134 6/134 0.17 [ 0.02, 1.37 ]<br />
Faye 2003 SEN 0/360 0/161 0.0 [ 0.0, 0.0 ]<br />
Mutabingwa 2004 TZA 0/515 1/519 0.34 [ 0.01, 8.23 ]<br />
Yeka 2004 UGA 4/731 12/730 0.33 [ 0.11, 1.03 ]<br />
Dorsey 2006 UGA 15/232 16/253 1.02 [ 0.52, 2.02 ]<br />
Kayentao 2006 MLI 0/133 0/132 0.0 [ 0.0, 0.0 ]<br />
Menard 2006 MDG 0/83 0/83 0.0 [ 0.0, 0.0 ]<br />
Total (95% CI) 2188 2012 0.61 [ 0.36, 1.03 ]<br />
Total events: 20 (AS+AQ), 35 (AQ+SP)<br />
Heterogeneity: Chi 2 = 4.92, df = 3 (P = 0.18); I 2 =39%<br />
Test <strong>for</strong> overall effect: Z = 1.84 (P = 0.065)<br />
0.01 0.1 1 10 100<br />
Favours ASAQ Favours AQSP<br />
Analysis 14.1. Comparison 14 Dihydroartemisinin-piperaquine dose analysis: 3 dose vs 4 dose regimen,<br />
Outcome 1 Total Failure PCR unadjusted.<br />
Review: <strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria<br />
Comparison: 14 Dihydroartemisinin-piperaquine dose analysis: 3 dose vs 4 dose regimen<br />
Outcome: 1 Total Failure PCR unadjusted<br />
Study or subgroup DHA-P (4 doses) DHA-P (3 doses) Risk Ratio Weight Risk Ratio<br />
1 Day 63<br />
n/N n/N M-H,Fixed,95% CI M-H,Fixed,95% CI<br />
Ashley 2004 THA 18/155 11/163 100.0 % 1.72 [ 0.84, 3.53 ]<br />
Total (95% CI) 155 163 100.0 % 1.72 [ 0.84, 3.53 ]<br />
Total events: 18 (DHA-P (4 doses)), 11 (DHA-P (3 doses))<br />
Heterogeneity: not applicable<br />
Test <strong>for</strong> overall effect: Z = 1.48 (P = 0.14)<br />
0.01 0.1 1 10 100<br />
Favours DHA-P (4 doses) Favours DHA-P (3 doses)<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
200