Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
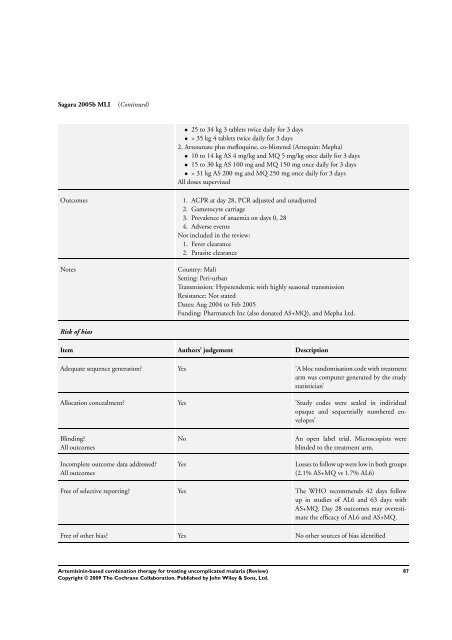
Sagara 2005b MLI (Continued)<br />
• 25 to 34 kg 3 tablets twice daily <strong>for</strong> 3 days<br />
• > 35 kg 4 tablets twice daily <strong>for</strong> 3 days<br />
2. Artesunate plus mefloquine, co-blistered (Artequin: Mepha)<br />
• 10 to 14 kg AS 4 mg/kg and MQ 5 mg/kg once daily <strong>for</strong> 3 days<br />
• 15 to 30 kg AS 100 mg and MQ 150 mg once daily <strong>for</strong> 3 days<br />
• > 31 kg AS 200 mg and MQ 250 mg once daily <strong>for</strong> 3 days<br />
All doses supervized<br />
Outcomes 1. ACPR at day 28, PCR adjusted and unadjusted<br />
2. Gametocyte carriage<br />
3. Prevalence of anaemia on days 0, 28<br />
4. Adverse events<br />
Not included in the review:<br />
1. Fever clearance<br />
2. Parasite clearance<br />
Notes Country: Mali<br />
Setting: Peri-urban<br />
Transmission: Hyperendemic with highly seasonal transmission<br />
Resistance: Not stated<br />
Dates: Aug 2004 to Feb 2005<br />
Funding: Pharmatech Inc (also donated AS+MQ), and Mepha Ltd.<br />
Risk of bias<br />
Item Authors’ judgement Description<br />
Adequate sequence generation? Yes ’A bloc randomisation code with treatment<br />
arm was computer generated by the study<br />
statistician’<br />
Allocation concealment? Yes ’Study codes were sealed in individual<br />
opaque and sequentially numbered envelopes’<br />
Blinding?<br />
All outcomes<br />
Incomplete outcome data addressed?<br />
All outcomes<br />
No An open label trial. Microscopists were<br />
blinded to the treatment arm.<br />
Yes Losses to follow up were low in both groups<br />
(2.1% AS+MQ vs 1.7% AL6)<br />
Free of selective reporting? Yes <strong>The</strong> WHO recommends 42 days follow<br />
up in studies of AL6 and 63 days with<br />
AS+MQ. Day 28 outcomes may overestimate<br />
the efficacy of AL6 and AS+MQ.<br />
Free of other bias? Yes No other sources of bias identified<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
87