Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
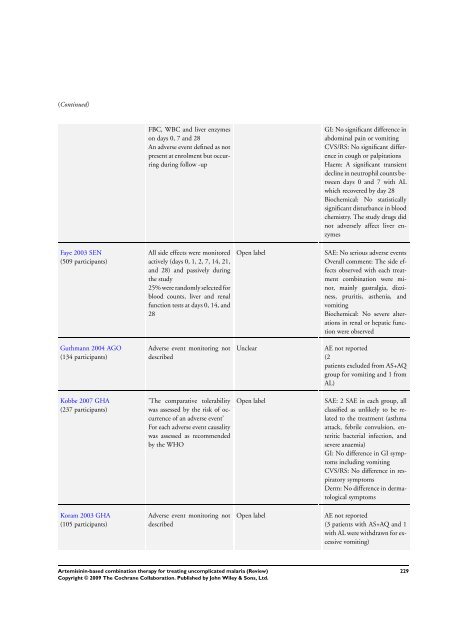
(Continued)<br />
Faye 2003 SEN<br />
(509 participants)<br />
Guthmann 2004 AGO<br />
(134 participants)<br />
Kobbe 2007 GHA<br />
(237 participants)<br />
Koram 2003 GHA<br />
(105 participants)<br />
FBC, WBC and liver enzymes<br />
on days 0, 7 and 28<br />
An adverse event defined as not<br />
present at enrolment but occurring<br />
during follow -up<br />
All side effects were monitored<br />
actively (days 0, 1, 2, 7, 14, 21,<br />
and 28) and passively during<br />
the study<br />
25% were randomly selected <strong>for</strong><br />
blood counts, liver and renal<br />
function tests at days 0, 14, and<br />
28<br />
Adverse event monitoring not<br />
described<br />
’<strong>The</strong> comparative tolerability<br />
was assessed by the risk of occurrence<br />
of an adverse event’<br />
For each adverse event causality<br />
was assessed as recommended<br />
by the WHO<br />
Adverse event monitoring not<br />
described<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
GI: No significant difference in<br />
abdominal pain or vomiting<br />
CVS/RS: No significant difference<br />
in cough or palpitations<br />
Haem: A significant transient<br />
decline in neutrophil counts between<br />
days 0 and 7 with AL<br />
which recovered by day 28<br />
Biochemical: No statistically<br />
significant disturbance in blood<br />
chemistry. <strong>The</strong> study drugs did<br />
not adversely affect liver enzymes<br />
Open label SAE: No serious adverse events<br />
Overall comment: <strong>The</strong> side effects<br />
observed with each treatment<br />
<strong>combination</strong> were minor,<br />
mainly gastralgia, dizziness,<br />
pruritis, asthenia, and<br />
vomiting<br />
Biochemical: No severe alterations<br />
in renal or hepatic function<br />
were observed<br />
Unclear AE not reported<br />
(2<br />
patients excluded from AS+AQ<br />
group <strong>for</strong> vomiting and 1 from<br />
AL)<br />
Open label SAE: 2 SAE in each group, all<br />
classified as unlikely to be related<br />
to the treatment (asthma<br />
attack, febrile convulsion, enteritic<br />
bacterial infection, and<br />
severe anaemia)<br />
GI: No difference in GI symptoms<br />
including vomiting<br />
CVS/RS: No difference in respiratory<br />
symptoms<br />
Derm: No difference in dermatological<br />
symptoms<br />
Open label AE not reported<br />
(3 patients with AS+AQ and 1<br />
with AL were withdrawn <strong>for</strong> excessive<br />
vomiting)<br />
229