Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
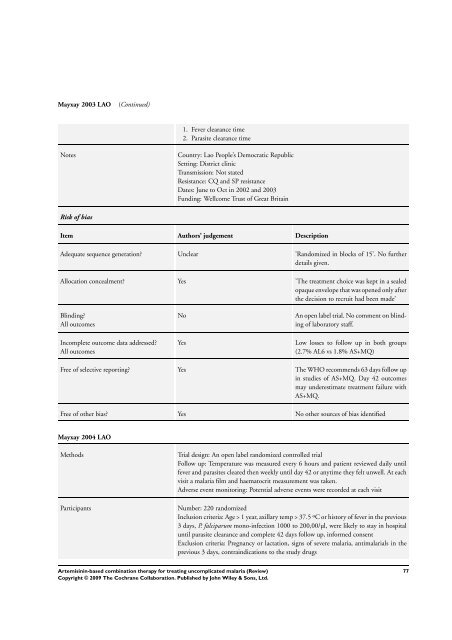
Mayxay 2003 LAO (Continued)<br />
1. Fever clearance time<br />
2. Parasite clearance time<br />
Notes Country: Lao People’s Democratic Republic<br />
Setting: District clinic<br />
Transmission: Not stated<br />
Resistance: CQ and SP resistance<br />
Dates: June to Oct in 2002 and 2003<br />
Funding: Wellcome Trust of Great Britain<br />
Risk of bias<br />
Item Authors’ judgement Description<br />
Adequate sequence generation? Unclear ’Randomized in blocks of 15’. No further<br />
details given.<br />
Allocation concealment? Yes ’<strong>The</strong> treatment choice was kept in a sealed<br />
opaque envelope that was opened only after<br />
the decision to recruit had been made’<br />
Blinding?<br />
All outcomes<br />
Incomplete outcome data addressed?<br />
All outcomes<br />
No An open label trial. No comment on blinding<br />
of laboratory staff.<br />
Yes Low losses to follow up in both groups<br />
(2.7% AL6 vs 1.8% AS+MQ)<br />
Free of selective reporting? Yes <strong>The</strong> WHO recommends 63 days follow up<br />
in studies of AS+MQ. Day 42 outcomes<br />
may underestimate treatment failure with<br />
AS+MQ.<br />
Free of other bias? Yes No other sources of bias identified<br />
Mayxay 2004 LAO<br />
Methods Trial design: An open label randomized controlled trial<br />
Follow up: Temperature was measured every 6 hours and patient reviewed daily until<br />
fever and parasites cleared then weekly until day 42 or anytime they felt unwell. At each<br />
visit a malaria film and haematocrit measurement was taken.<br />
Adverse event monitoring: Potential adverse events were recorded at each visit<br />
Participants Number: 220 randomized<br />
Inclusion criteria: Age > 1 year, axillary temp > 37.5 ºC or history of fever in the previous<br />
3 days, P. falciparum mono-infection 1000 to 200,00/µl, were likely to stay in hospital<br />
until parasite clearance and complete 42 days follow up, in<strong>for</strong>med consent<br />
Exclusion criteria: Pregnancy or lactation, signs of severe malaria, antimalarials in the<br />
previous 3 days, contraindications to the study drugs<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
77