Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
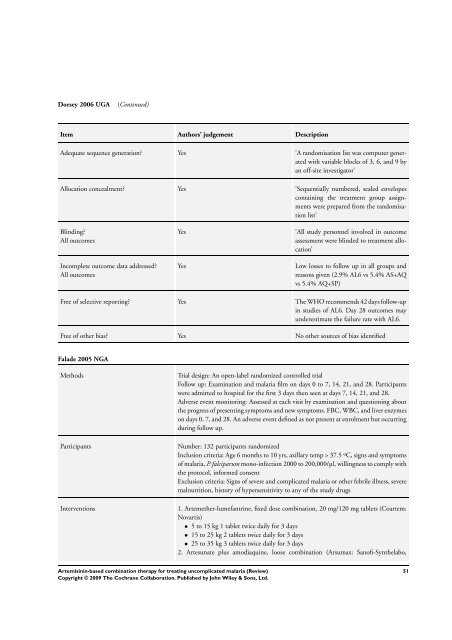
Dorsey 2006 UGA (Continued)<br />
Item Authors’ judgement Description<br />
Adequate sequence generation? Yes ’A randomisation list was computer generated<br />
with variable blocks of 3, 6, and 9 by<br />
an off-site investigator’<br />
Allocation concealment? Yes ’Sequentially numbered, sealed envelopes<br />
containing the treatment group assignments<br />
were prepared from the randomisation<br />
list’<br />
Blinding?<br />
All outcomes<br />
Incomplete outcome data addressed?<br />
All outcomes<br />
Yes ’All study personnel involved in outcome<br />
assessment were blinded to treatment allocation’<br />
Yes Low losses to follow up in all groups and<br />
reasons given (2.9% AL6 vs 5.4% AS+AQ<br />
vs 5.4% AQ+SP)<br />
Free of selective reporting? Yes <strong>The</strong> WHO recommends 42 days follow-up<br />
in studies of AL6. Day 28 outcomes may<br />
underestimate the failure rate with AL6.<br />
Free of other bias? Yes No other sources of bias identified<br />
Falade 2005 NGA<br />
Methods Trial design: An open-label randomized controlled trial<br />
Follow up: Examination and malaria film on days 0 to 7, 14, 21, and 28. Participants<br />
were admitted to hospital <strong>for</strong> the first 3 days then seen at days 7, 14, 21, and 28.<br />
Adverse event monitoring: Assessed at each visit by examination and questioning about<br />
the progress of presenting symptoms and new symptoms. FBC, WBC, and liver enzymes<br />
on days 0, 7, and 28. An adverse event defined as not present at enrolment but occurring<br />
during follow up.<br />
Participants Number: 132 participants randomized<br />
Inclusion criteria: Age 6 months to 10 yrs, axillary temp > 37.5 ºC, signs and symptoms<br />
of malaria, P. falciparum mono-infection 2000 to 200,000/µl, willingness to comply with<br />
the protocol, in<strong>for</strong>med consent<br />
Exclusion criteria: Signs of severe and complicated malaria or other febrile illness, severe<br />
malnutrition, history of hypersensitivity to any of the study drugs<br />
Interventions 1. Artemether-lumefantrine, fixed dose <strong>combination</strong>, 20 mg/120 mg tablets (Coartem:<br />
Novartis)<br />
• 5 to 15 kg 1 tablet twice daily <strong>for</strong> 3 days<br />
• 15 to 25 kg 2 tablets twice daily <strong>for</strong> 3 days<br />
• 25 to 35 kg 3 tablets twice daily <strong>for</strong> 3 days<br />
2. Artesunate plus amodiaquine, loose <strong>combination</strong> (Arsumax: Sanofi-Synthelabo,<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
51