Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
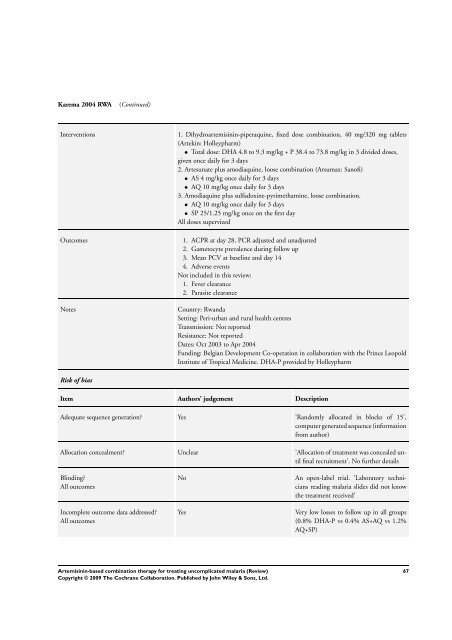
Karema 2004 RWA (Continued)<br />
Interventions 1. Dihydroartemisinin-piperaquine, fixed dose <strong>combination</strong>, 40 mg/320 mg tablets<br />
(Artekin: Holleypharm)<br />
• Total dose: DHA 4.8 to 9.3 mg/kg + P 38.4 to 73.8 mg/kg in 3 divided doses,<br />
given once daily <strong>for</strong> 3 days<br />
2. Artesunate plus amodiaquine, loose <strong>combination</strong> (Arsumax: Sanofi)<br />
• AS 4 mg/kg once daily <strong>for</strong> 3 days<br />
• AQ 10 mg/kg once daily <strong>for</strong> 3 days<br />
3. Amodiaquine plus sulfadoxine-pyrimethamine, loose <strong>combination</strong>.<br />
• AQ 10 mg/kg once daily <strong>for</strong> 3 days<br />
• SP 25/1.25 mg/kg once on the first day<br />
All doses supervized<br />
Outcomes 1. ACPR at day 28, PCR adjusted and unadjusted<br />
2. Gametocyte prevalence during follow up<br />
3. Mean PCV at baseline and day 14<br />
4. Adverse events<br />
Not included in this review:<br />
1. Fever clearance<br />
2. Parasite clearance<br />
Notes Country: Rwanda<br />
Setting: Peri-urban and rural health centres<br />
Transmission: Not reported<br />
Resistance: Not reported<br />
Dates: Oct 2003 to Apr 2004<br />
Funding: Belgian Development Co-operation in collaboration with the Prince Leopold<br />
Institute of Tropical Medicine. DHA-P provided by Holleypharm<br />
Risk of bias<br />
Item Authors’ judgement Description<br />
Adequate sequence generation? Yes ’Randomly allocated in blocks of 15’,<br />
computer generated sequence (in<strong>for</strong>mation<br />
from author)<br />
Allocation concealment? Unclear ’Allocation of treatment was concealed until<br />
final recruitment’. No further details<br />
Blinding?<br />
All outcomes<br />
Incomplete outcome data addressed?<br />
All outcomes<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
No An open-label trial. ’Laboratory technicians<br />
reading malaria slides did not know<br />
the treatment received’<br />
Yes Very low losses to follow up in all groups<br />
(0.8% DHA-P vs 0.4% AS+AQ vs 1.2%<br />
AQ+SP)<br />
67