Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
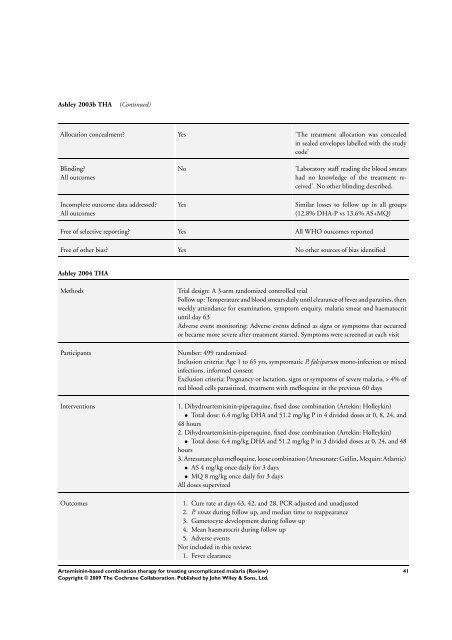
Ashley 2003b THA (Continued)<br />
Allocation concealment? Yes ’<strong>The</strong> treatment allocation was concealed<br />
in sealed envelopes labelled with the study<br />
code’<br />
Blinding?<br />
All outcomes<br />
Incomplete outcome data addressed?<br />
All outcomes<br />
No ’Laboratory staff reading the blood smears<br />
had no knowledge of the treatment received’.<br />
No other blinding described.<br />
Yes Similar losses to follow up in all groups<br />
(12.8% DHA-P vs 13.6% AS+MQ)<br />
Free of selective reporting? Yes All WHO outcomes reported<br />
Free of other bias? Yes No other sources of bias identified<br />
Ashley 2004 THA<br />
Methods Trial design: A 3-arm randomized controlled trial<br />
Follow up: Temperature and blood smears daily until clearance of fever and parasites, then<br />
weekly attendance <strong>for</strong> examination, symptom enquiry, malaria smear and haematocrit<br />
until day 63<br />
Adverse event monitoring: Adverse events defined as signs or symptoms that occurred<br />
or became more severe after treatment started. Symptoms were screened at each visit<br />
Participants Number: 499 randomized<br />
Inclusion criteria: Age 1 to 65 yrs, symptomatic P. falciparum mono-infection or mixed<br />
infections, in<strong>for</strong>med consent<br />
Exclusion criteria: Pregnancy or lactation, signs or symptoms of severe malaria, > 4% of<br />
red blood cells parasitized, treatment with mefloquine in the previous 60 days<br />
Interventions 1. Dihydroartemisinin-piperaquine, fixed dose <strong>combination</strong> (Artekin: Holleykin)<br />
• Total dose: 6.4 mg/kg DHA and 51.2 mg/kg P in 4 divided doses at 0, 8, 24, and<br />
48 hours<br />
2. Dihydroartemisinin-piperaquine, fixed dose <strong>combination</strong> (Artekin: Holleykin)<br />
• Total dose: 6.4 mg/kg DHA and 51.2 mg/kg P in 3 divided doses at 0, 24, and 48<br />
hours<br />
3. Artesunate plus mefloquine, loose <strong>combination</strong> (Artesunate: Guilin, Mequin: Atlantic)<br />
• AS 4 mg/kg once daily <strong>for</strong> 3 days<br />
• MQ 8 mg/kg once daily <strong>for</strong> 3 days<br />
All doses supervized<br />
Outcomes 1. Cure rate at days 63, 42, and 28, PCR adjusted and unadjusted<br />
2. P. vivax during follow up, and median time to reappearance<br />
3. Gametocyte development during follow up<br />
4. Mean haematocrit during follow up<br />
5. Adverse events<br />
Not included in this review:<br />
1. Fever clearance<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
41