Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
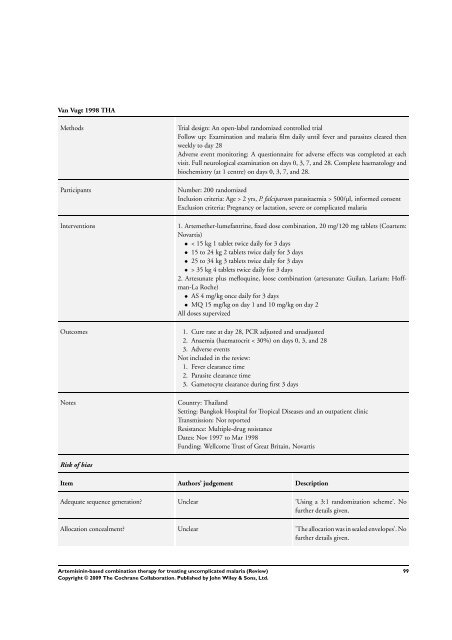
Van Vugt 1998 THA<br />
Methods Trial design: An open-label randomized controlled trial<br />
Follow up: Examination and malaria film daily until fever and parasites cleared then<br />
weekly to day 28<br />
Adverse event monitoring: A questionnaire <strong>for</strong> adverse effects was completed at each<br />
visit. Full neurological examination on days 0, 3, 7, and 28. Complete haematology and<br />
biochemistry (at 1 centre) on days 0, 3, 7, and 28.<br />
Participants Number: 200 randomized<br />
Inclusion criteria: Age > 2 yrs, P. falciparum parasitaemia > 500/µl, in<strong>for</strong>med consent<br />
Exclusion criteria: Pregnancy or lactation, severe or complicated malaria<br />
Interventions 1. Artemether-lumefantrine, fixed dose <strong>combination</strong>, 20 mg/120 mg tablets (Coartem:<br />
Novartis)<br />
• < 15 kg 1 tablet twice daily <strong>for</strong> 3 days<br />
• 15 to 24 kg 2 tablets twice daily <strong>for</strong> 3 days<br />
• 25 to 34 kg 3 tablets twice daily <strong>for</strong> 3 days<br />
• > 35 kg 4 tablets twice daily <strong>for</strong> 3 days<br />
2. Artesunate plus mefloquine, loose <strong>combination</strong> (artesunate: Guilan, Lariam: Hoffman-La<br />
Roche)<br />
• AS 4 mg/kg once daily <strong>for</strong> 3 days<br />
• MQ 15 mg/kg on day 1 and 10 mg/kg on day 2<br />
All doses supervized<br />
Outcomes 1. Cure rate at day 28, PCR adjusted and unadjusted<br />
2. Anaemia (haematocrit < 30%) on days 0, 3, and 28<br />
3. Adverse events<br />
Not included in the review:<br />
1. Fever clearance time<br />
2. Parasite clearance time<br />
3. Gametocyte clearance during first 3 days<br />
Notes Country: Thailand<br />
Setting: Bangkok Hospital <strong>for</strong> Tropical Diseases and an outpatient clinic<br />
Transmission: Not reported<br />
Resistance: Multiple-drug resistance<br />
Dates: Nov 1997 to Mar 1998<br />
Funding: Wellcome Trust of Great Britain, Novartis<br />
Risk of bias<br />
Item Authors’ judgement Description<br />
Adequate sequence generation? Unclear ’Using a 3:1 randomization scheme’. No<br />
further details given.<br />
Allocation concealment? Unclear ’<strong>The</strong> allocation was in sealed envelopes’. No<br />
further details given.<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
99