Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
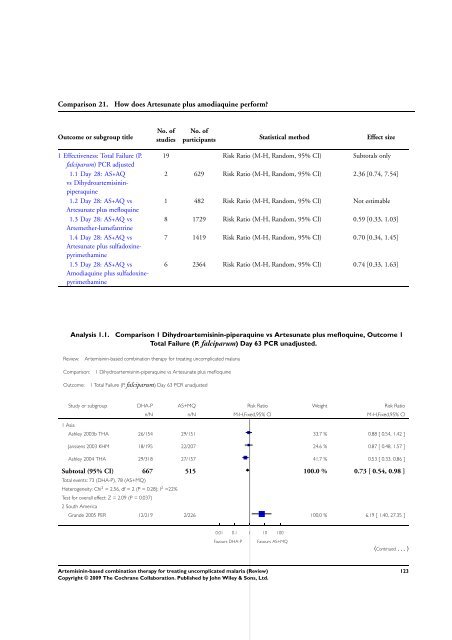
Comparison 21. How does Artesunate plus amodiaquine per<strong>for</strong>m?<br />
Outcome or subgroup title<br />
1 Effectiveness: Total Failure (P.<br />
falciparum) PCR adjusted<br />
1.1 Day 28: AS+AQ<br />
vs Dihydroartemisininpiperaquine<br />
1.2 Day 28: AS+AQ vs<br />
Artesunate plus mefloquine<br />
1.3 Day 28: AS+AQ vs<br />
Artemether-lumefantrine<br />
1.4 Day 28: AS+AQ vs<br />
Artesunate plus sulfadoxinepyrimethamine<br />
1.5 Day 28: AS+AQ vs<br />
Amodiaquine plus sulfadoxinepyrimethamine<br />
No. of<br />
studies<br />
No. of<br />
participants Statistical method Effect size<br />
19 Risk Ratio (M-H, Random, 95% CI) Subtotals only<br />
2 629 Risk Ratio (M-H, Random, 95% CI) 2.36 [0.74, 7.54]<br />
1 482 Risk Ratio (M-H, Random, 95% CI) Not estimable<br />
8 1729 Risk Ratio (M-H, Random, 95% CI) 0.59 [0.33, 1.03]<br />
7 1419 Risk Ratio (M-H, Random, 95% CI) 0.70 [0.34, 1.45]<br />
6 2364 Risk Ratio (M-H, Random, 95% CI) 0.74 [0.33, 1.63]<br />
Analysis 1.1. Comparison 1 Dihydroartemisinin-piperaquine vs Artesunate plus mefloquine, Outcome 1<br />
Total Failure (P. falciparum) Day 63 PCR unadjusted.<br />
Review: <strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria<br />
Comparison: 1 Dihydroartemisinin-piperaquine vs Artesunate plus mefloquine<br />
Outcome: 1 Total Failure (P. falciparum) Day 63 PCR unadjusted<br />
1 Asia<br />
Study or subgroup DHA-P AS+MQ Risk Ratio Weight Risk Ratio<br />
n/N n/N M-H,Fixed,95% CI M-H,Fixed,95% CI<br />
Ashley 2003b THA 26/154 29/151 33.7 % 0.88 [ 0.54, 1.42 ]<br />
Janssens 2003 KHM 18/195 22/207 24.6 % 0.87 [ 0.48, 1.57 ]<br />
Ashley 2004 THA 29/318 27/157 41.7 % 0.53 [ 0.33, 0.86 ]<br />
Subtotal (95% CI) 667 515 100.0 % 0.73 [ 0.54, 0.98 ]<br />
Total events: 73 (DHA-P), 78 (AS+MQ)<br />
Heterogeneity: Chi 2 = 2.56, df = 2 (P = 0.28); I 2 =22%<br />
Test <strong>for</strong> overall effect: Z = 2.09 (P = 0.037)<br />
2 South America<br />
Grande 2005 PER 12/219 2/226 100.0 % 6.19 [ 1.40, 27.35 ]<br />
0.01 0.1 1 10 100<br />
Favours DHA-P Favours AS+MQ<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
(Continued ...)<br />
123