Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
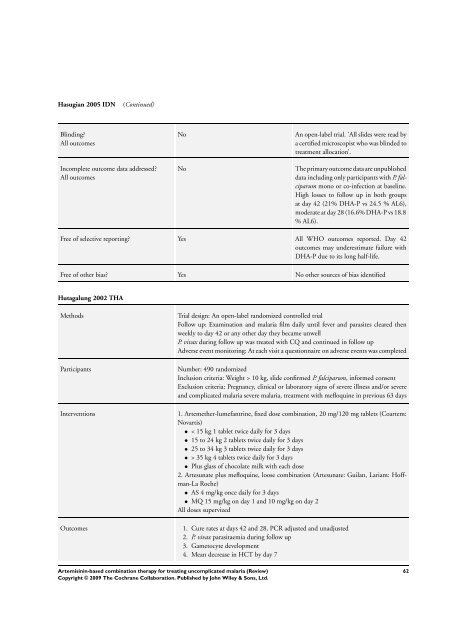
Hasugian 2005 IDN (Continued)<br />
Blinding?<br />
All outcomes<br />
Incomplete outcome data addressed?<br />
All outcomes<br />
No An open-label trial. ’All slides were read by<br />
a certified microscopist who was blinded to<br />
treatment allocation’.<br />
No <strong>The</strong> primary outcome data are unpublished<br />
data including only participants with P. falciparum<br />
mono or co-infection at baseline.<br />
High losses to follow up in both groups<br />
at day 42 (21% DHA-P vs 24.5 % AL6),<br />
moderate at day 28 (16.6% DHA-P vs 18.8<br />
% AL6).<br />
Free of selective reporting? Yes All WHO outcomes reported. Day 42<br />
outcomes may underestimate failure with<br />
DHA-P due to its long half-life.<br />
Free of other bias? Yes No other sources of bias identified<br />
Hutagalung 2002 THA<br />
Methods Trial design: An open-label randomized controlled trial<br />
Follow up: Examination and malaria film daily until fever and parasites cleared then<br />
weekly to day 42 or any other day they became unwell<br />
P. vivax during follow up was treated with CQ and continued in follow up<br />
Adverse event monitoring: At each visit a questionnaire on adverse events was completed<br />
Participants Number: 490 randomized<br />
Inclusion criteria: Weight > 10 kg, slide confirmed P. falciparum, in<strong>for</strong>med consent<br />
Exclusion criteria: Pregnancy, clinical or laboratory signs of severe illness and/or severe<br />
and complicated malaria severe malaria, treatment with mefloquine in previous 63 days<br />
Interventions 1. Artemether-lumefantrine, fixed dose <strong>combination</strong>, 20 mg/120 mg tablets (Coartem:<br />
Novartis)<br />
• < 15 kg 1 tablet twice daily <strong>for</strong> 3 days<br />
• 15 to 24 kg 2 tablets twice daily <strong>for</strong> 3 days<br />
• 25 to 34 kg 3 tablets twice daily <strong>for</strong> 3 days<br />
• > 35 kg 4 tablets twice daily <strong>for</strong> 3 days<br />
• Plus glass of chocolate milk with each dose<br />
2. Artesunate plus mefloquine, loose <strong>combination</strong> (Artesunate: Guilan, Lariam: Hoffman-La<br />
Roche)<br />
• AS 4 mg/kg once daily <strong>for</strong> 3 days<br />
• MQ 15 mg/kg on day 1 and 10 mg/kg on day 2<br />
All doses supervized<br />
Outcomes 1. Cure rates at days 42 and 28, PCR adjusted and unadjusted<br />
2. P. vivax parasitaemia during follow up<br />
3. Gametocyte development<br />
4. Mean decrease in HCT by day 7<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
62