Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
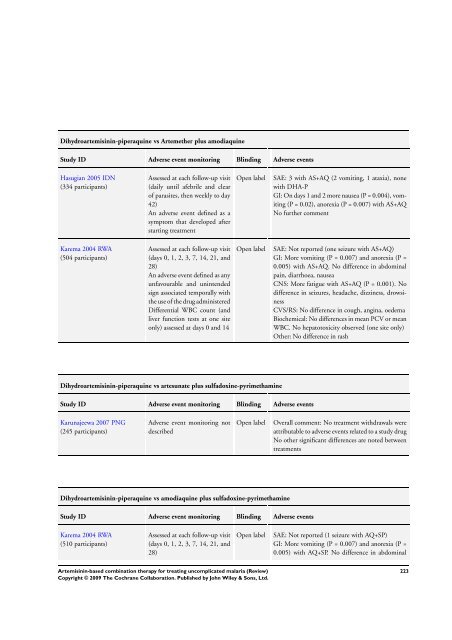
Dihydroartemisinin-piperaquine vs Artemether plus amodiaquine<br />
Study ID Adverse event monitoring Blinding Adverse events<br />
Hasugian 2005 IDN<br />
(334 participants)<br />
Karema 2004 RWA<br />
(504 participants)<br />
Assessed at each follow-up visit<br />
(daily until afebrile and clear<br />
of parasites, then weekly to day<br />
42)<br />
An adverse event defined as a<br />
symptom that developed after<br />
starting treatment<br />
Assessed at each follow-up visit<br />
(days 0, 1, 2, 3, 7, 14, 21, and<br />
28)<br />
An adverse event defined as any<br />
unfavourable and unintended<br />
sign associated temporally with<br />
the use of the drug administered<br />
Differential WBC count (and<br />
liver function tests at one site<br />
only) assessed at days 0 and 14<br />
Dihydroartemisinin-piperaquine vs artesunate plus sulfadoxine-pyrimethamine<br />
Study ID Adverse event monitoring Blinding Adverse events<br />
Karunajeewa 2007 PNG<br />
(245 participants)<br />
Adverse event monitoring not<br />
described<br />
Dihydroartemisinin-piperaquine vs amodiaquine plus sulfadoxine-pyrimethamine<br />
Study ID Adverse event monitoring Blinding Adverse events<br />
Karema 2004 RWA<br />
(510 participants)<br />
Assessed at each follow-up visit<br />
(days 0, 1, 2, 3, 7, 14, 21, and<br />
28)<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
Open label SAE: 3 with AS+AQ (2 vomiting, 1 ataxia), none<br />
with DHA-P<br />
GI: On days 1 and 2 more nausea (P = 0.004), vomiting<br />
(P = 0.02), anorexia (P = 0.007) with AS+AQ<br />
No further comment<br />
Open label SAE: Not reported (one seizure with AS+AQ)<br />
GI: More vomiting (P = 0.007) and anorexia (P =<br />
0.005) with AS+AQ. No difference in abdominal<br />
pain, diarrhoea, nausea<br />
CNS: More fatigue with AS+AQ (P = 0.001). No<br />
difference in seizures, headache, dizziness, drowsiness<br />
CVS/RS: No difference in cough, angina, oedema<br />
Biochemical: No differences in mean PCV or mean<br />
WBC. No hepatotoxicity observed (one site only)<br />
Other: No difference in rash<br />
Open label Overall comment: No treatment withdrawals were<br />
attributable to adverse events related to a study drug<br />
No other significant differences are noted between<br />
treatments<br />
Open label SAE: Not reported (1 seizure with AQ+SP)<br />
GI: More vomiting (P = 0.007) and anorexia (P =<br />
0.005) with AQ+SP. No difference in abdominal<br />
223