- Page 1 and 2:
Artemisinin-based combination thera
- Page 3 and 4:
Analysis 2.2. Comparison 2 Dihydroa
- Page 5 and 6:
Analysis 10.5. Comparison 10 Arteme
- Page 7 and 8:
[Intervention Review] Artemisinin-b
- Page 9 and 10:
B A C K G R O U N D Malaria is a di
- Page 11 and 12:
drugs and the treatment comparisons
- Page 13 and 14:
this analysis into participants who
- Page 15 and 16:
Figure 1. Methodological quality su
- Page 17 and 18:
confidence intervals of the estimat
- Page 19 and 20:
Anaemia Hasugian 2005 IDN found tha
- Page 21 and 22:
Three trials report significantly m
- Page 23 and 24:
or those treated for P. vivax at ba
- Page 25 and 26:
Early vomiting Not reported. D I S
- Page 27 and 28:
Figure 4. Olliaro-Vaillant plot. Da
- Page 29 and 30:
Figure 6. Olliaro-Vaillant plot. Da
- Page 31 and 32:
Figure 8. Olliaro-Vaillant plot. Da
- Page 33 and 34:
Figure 10. Olliaro-Vaillant plot. D
- Page 35 and 36:
of findings tables’. For these ta
- Page 37 and 38:
Huambo and Bie provinces, central A
- Page 39 and 40:
References to studies excluded from
- Page 41 and 42:
Adjuik 2004 Adjuik M, Babiker A, Ga
- Page 43 and 44:
C H A R A C T E R I S T I C S O F S
- Page 45 and 46:
Ashley 2003a THA (Continued) Outcom
- Page 47 and 48:
Ashley 2003b THA (Continued) Alloca
- Page 49 and 50:
Ashley 2005 THA (Continued) Exclusi
- Page 51 and 52:
Bonnet 2004 GIN (Continued) Item Au
- Page 53 and 54:
Bukirwa 2005 UGA Methods Trial desi
- Page 55 and 56:
Djimde 2004 MLI (Continued) • AS
- Page 57 and 58:
Dorsey 2006 UGA (Continued) Item Au
- Page 59 and 60:
Fanello 2004 RWA Methods Trial desi
- Page 61 and 62:
Faye 2003 SEN (Continued) Outcomes
- Page 63 and 64:
Grande 2005 PER (Continued) Allocat
- Page 65 and 66:
Guthmann 2004 AGO (Continued) All d
- Page 67 and 68:
Hasugian 2005 IDN Methods Trial des
- Page 69 and 70:
Hutagalung 2002 THA (Continued) 5.
- Page 71 and 72:
Janssens 2003 KHM (Continued) Free
- Page 73 and 74:
Karema 2004 RWA (Continued) Interve
- Page 75 and 76:
Karunajeewa 2007 PNG (Continued) Ri
- Page 77 and 78:
Kobbe 2007 GHA (Continued) Exclusio
- Page 79 and 80:
Koram 2003 GHA (Continued) Adequate
- Page 81 and 82:
Martensson 2003 TZA Methods Trial d
- Page 83 and 84:
Mayxay 2003 LAO (Continued) 1. Feve
- Page 85 and 86:
Menard 2006 MDG Methods Trial desig
- Page 87 and 88:
Mens 2007 KEN (Continued) Risk of b
- Page 89 and 90:
Mutabingwa 2004 TZA (Continued) •
- Page 91 and 92:
Owusu-Agyei 2006 GHA (Continued) Fr
- Page 93 and 94:
Sagara 2005b MLI (Continued) • 25
- Page 95 and 96:
Smithuis 2004 MMR (Continued) Risk
- Page 97 and 98:
Stohrer 2003 LAO Methods Trial desi
- Page 99 and 100:
Swarthout 2004 ZAR (Continued) Note
- Page 101 and 102:
Tran 2002 VNM Methods Trial design:
- Page 103 and 104:
Van den Broek 2003a BGD (Continued)
- Page 105 and 106:
Van Vugt 1998 THA Methods Trial des
- Page 107 and 108:
Yeka 2004 UGA (Continued) Risk of b
- Page 109 and 110:
Yeka 2007 UGA (Continued) Free of o
- Page 111 and 112:
Zongo 2007 BFA (Continued) Novartis
- Page 113 and 114:
(Continued) Fofana 2005 Conference
- Page 115 and 116:
D A T A A N D A N A L Y S E S Compa
- Page 117 and 118:
2.2 Asia 1 317 Risk Ratio (M-H, Fix
- Page 119 and 120:
Comparison 5. Dihydroartemisinin-pi
- Page 121 and 122:
Comparison 8. Artesunate plus meflo
- Page 123 and 124:
Comparison 10. Artemether-lumefantr
- Page 125 and 126:
Comparison 12. Artesunate plus amod
- Page 127 and 128:
5.2 DHA-P 3 doses 3 1116 Risk Ratio
- Page 129 and 130:
Comparison 21. How does Artesunate
- Page 131 and 132:
Analysis 1.3. Comparison 1 Dihydroa
- Page 133 and 134:
Analysis 1.7. Comparison 1 Dihydroa
- Page 135 and 136:
Analysis 1.8. Comparison 1 Dihydroa
- Page 137 and 138:
(... Continued) Study or subgroup D
- Page 139 and 140:
Analysis 1.12. Comparison 1 Dihydro
- Page 141 and 142:
(... Continued) Study or subgroup D
- Page 143 and 144:
Analysis 2.2. Comparison 2 Dihydroa
- Page 145 and 146:
Analysis 2.4. Comparison 2 Dihydroa
- Page 147 and 148:
Analysis 2.6. Comparison 2 Dihydroa
- Page 149 and 150:
Analysis 2.8. Comparison 2 Dihydroa
- Page 151 and 152:
Analysis 3.2. Comparison 3 Dihydroa
- Page 153 and 154:
Analysis 3.5. Comparison 3 Dihydroa
- Page 155 and 156:
Analysis 4.1. Comparison 4 Dihydroa
- Page 157 and 158:
Analysis 4.5. Comparison 4 Dihydroa
- Page 159 and 160:
Analysis 5.4. Comparison 5 Dihydroa
- Page 161 and 162:
Analysis 5.7. Comparison 5 Dihydroa
- Page 163 and 164:
(... Continued) Study or subgroup A
- Page 165 and 166:
Analysis 6.6. Comparison 6 Artesuna
- Page 167 and 168:
Analysis 6.8. Comparison 6 Artesuna
- Page 169 and 170:
Analysis 7.2. Comparison 7 Artesuna
- Page 171 and 172:
Analysis 8.2. Comparison 8 Artesuna
- Page 173 and 174:
Analysis 9.2. Comparison 9 Artemeth
- Page 175 and 176:
(... Continued) Study or subgroup A
- Page 177 and 178:
Analysis 9.8. Comparison 9 Artemeth
- Page 179 and 180:
(... Continued) Study or subgroup A
- Page 181 and 182:
(... Continued) Study or subgroup A
- Page 183 and 184:
Analysis 10.2. Comparison 10 Arteme
- Page 185 and 186:
Analysis 10.4. Comparison 10 Arteme
- Page 187 and 188:
Analysis 10.6. Comparison 10 Arteme
- Page 189 and 190:
(... Continued) Study or subgroup A
- Page 191 and 192:
Analysis 11.3. Comparison 11 Arteme
- Page 193 and 194:
(... Continued) Study or subgroup A
- Page 195 and 196:
Analysis 11.9. Comparison 11 Arteme
- Page 197 and 198:
Analysis 12.2. Comparison 12 Artesu
- Page 199 and 200:
Analysis 12.4. Comparison 12 Artesu
- Page 201 and 202:
Analysis 13.1. Comparison 13 Artesu
- Page 203 and 204:
Analysis 13.3. Comparison 13 Artesu
- Page 205 and 206:
Analysis 13.5. Comparison 13 Artesu
- Page 207 and 208:
Analysis 14.2. Comparison 14 Dihydr
- Page 209 and 210: Analysis 15.2. Comparison 15 Dihydr
- Page 211 and 212: Analysis 15.4. Comparison 15 Dihydr
- Page 213 and 214: Analysis 15.6. Comparison 15 Dihydr
- Page 215 and 216: Analysis 17.1. Comparison 17 Artesu
- Page 217 and 218: (... Continued) Study or subgroup D
- Page 219 and 220: Analysis 20.1. Comparison 20 How do
- Page 221 and 222: Analysis 21.1. Comparison 21 How do
- Page 223 and 224: (Continued) 2. How does artesunate
- Page 225 and 226: (Continued) Sensitivity analysis 1
- Page 227 and 228: (Continued) Smithuis 2004 MMR (652
- Page 229 and 230: Dihydroartemisinin-piperaquine vs A
- Page 231 and 232: (Continued) Mayxay 2003 LAO (220 pa
- Page 233 and 234: Artesunate plus mefloquine vs Amodi
- Page 235 and 236: (Continued) Faye 2003 SEN (509 part
- Page 237 and 238: Artemether-lumefantrine vs Artesuna
- Page 239 and 240: (Continued) Zongo 2005 BFA (521 par
- Page 241 and 242: (Continued) Faye 2003 SEN (521 part
- Page 243 and 244: PCR = polymerase chain reaction PCV
- Page 245 and 246: (Continued) Proportion with anaemia
- Page 247 and 248: (Continued) Vivax efficacy: P. viva
- Page 249 and 250: (Continued) Efficacy: Total Failure
- Page 251 and 252: (Continued) Outcomes Illustrative c
- Page 253 and 254: (Continued) Efficacy: Total Failure
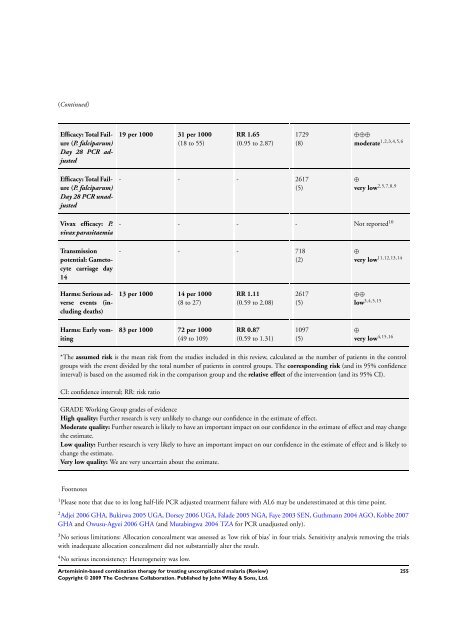
- Page 255 and 256: (Continued) GRADE Working Group gra
- Page 257 and 258: Footnotes 1 Data were also availabl
- Page 259: 8 Very serious imprecision: There w
- Page 263 and 264: (Continued) justed Vivax efficacy:
- Page 265 and 266: (Continued) Harms: Serious adverse
- Page 267 and 268: (Continued) Harms: Early vomiting -
- Page 269 and 270: 1 Dorsey 2006 UGA; Faye 2003 SEN; K