Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
(Continued)<br />
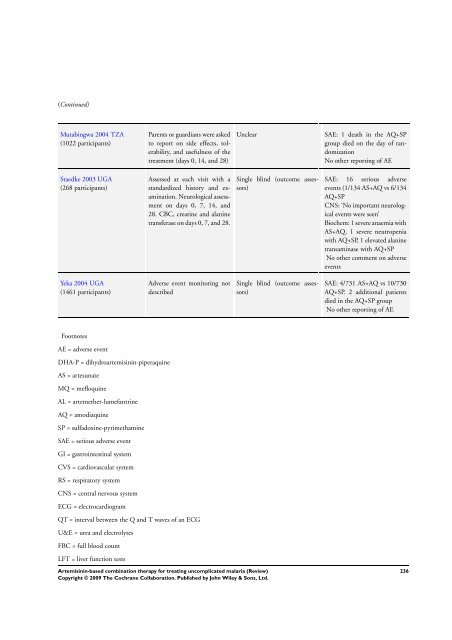
Mutabingwa 2004 TZA<br />
(1022 participants)<br />
Staedke 2003 UGA<br />
(268 participants)<br />
Yeka 2004 UGA<br />
(1461 participants)<br />
Footnotes<br />
AE = adverse event<br />
DHA-P = dihydroartemisinin-piperaquine<br />
AS = artesunate<br />
MQ = mefloquine<br />
AL = artemether-lumefantrine<br />
AQ = amodiaquine<br />
SP = sulfadoxine-pyrimethamine<br />
SAE = serious adverse event<br />
GI = gastrointestinal system<br />
CVS = cardiovascular system<br />
RS = respiratory system<br />
CNS = central nervous system<br />
ECG = electrocardiogram<br />
Parents or guardians were asked<br />
to report on side effects, tolerability,<br />
and usefulness of the<br />
treatment (days 0, 14, and 28)<br />
Assessed at each visit with a<br />
standardized history and examination.<br />
Neurological assessment<br />
on days 0, 7, 14, and<br />
28. CBC, creatine and alanine<br />
transferase on days 0, 7, and 28.<br />
Adverse event monitoring not<br />
described<br />
QT = interval between the Q and T waves of an ECG<br />
U&E = urea and electrolytes<br />
FBC = full blood count<br />
LFT = liver function tests<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
Unclear SAE: 1 death in the AQ+SP<br />
group died on the day of randomization<br />
No other reporting of AE<br />
Single blind (outcome assessors)<br />
Single blind (outcome assessors)<br />
SAE: 16 serious adverse<br />
events (1/134 AS+AQ vs 6/134<br />
AQ+SP<br />
CNS: ‘No important neurological<br />
events were seen’<br />
Biochem: 1 severe anaemia with<br />
AS+AQ, 1 severe neutropenia<br />
with AQ+SP, 1 elevated alanine<br />
transaminase with AQ+SP<br />
No other comment on adverse<br />
events<br />
SAE: 4/731 AS+AQ vs 10/730<br />
AQ+SP. 2 additional patients<br />
died in the AQ+SP group<br />
No other reporting of AE<br />
236