Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
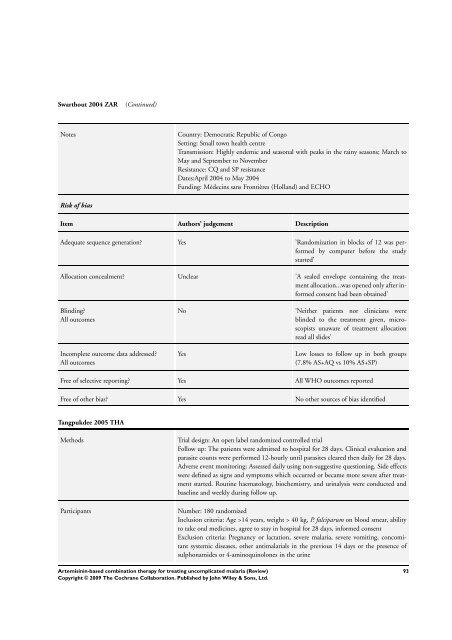
Swarthout 2004 ZAR (Continued)<br />
Notes Country: Democratic Republic of Congo<br />
Setting: Small town health centre<br />
Transmission: Highly endemic and seasonal with peaks in the rainy seasons; March to<br />
May and September to November<br />
Resistance: CQ and SP resistance<br />
Dates:April 2004 to May 2004<br />
Funding: Médecins sans Frontières (Holland) and ECHO<br />
Risk of bias<br />
Item Authors’ judgement Description<br />
Adequate sequence generation? Yes ’Randomization in blocks of 12 was per<strong>for</strong>med<br />
by computer be<strong>for</strong>e the study<br />
started’<br />
Allocation concealment? Unclear ’A sealed envelope containing the treatment<br />
allocation...was opened only after in<strong>for</strong>med<br />
consent had been obtained’<br />
Blinding?<br />
All outcomes<br />
Incomplete outcome data addressed?<br />
All outcomes<br />
No ’Neither patients nor clinicians were<br />
blinded to the treatment given, microscopists<br />
unaware of treatment allocation<br />
read all slides’<br />
Yes Low losses to follow up in both groups<br />
(7.8% AS+AQ vs 10% AS+SP)<br />
Free of selective reporting? Yes All WHO outcomes reported<br />
Free of other bias? Yes No other sources of bias identified<br />
Tangpukdee 2005 THA<br />
Methods Trial design: An open label randomized controlled trial<br />
Follow up: <strong>The</strong> patients were admitted to hospital <strong>for</strong> 28 days. Clinical evaluation and<br />
parasite counts were per<strong>for</strong>med 12-hourly until parasites cleared then daily <strong>for</strong> 28 days.<br />
Adverse event monitoring: Assessed daily using non-suggestive questioning. Side effects<br />
were defined as signs and symptoms which occurred or became more severe after treatment<br />
started. Routine haematology, biochemistry, and urinalysis were conducted and<br />
baseline and weekly during follow up.<br />
Participants Number: 180 randomized<br />
Inclusion criteria: Age >14 years, weight > 40 kg, P. falciparum on blood smear, ability<br />
to take oral medicines, agree to stay in hospital <strong>for</strong> 28 days, in<strong>for</strong>med consent<br />
Exclusion criteria: Pregnancy or lactation, severe malaria, severe vomiting, concomitant<br />
systemic diseases, other antimalarials in the previous 14 days or the presence of<br />
sulphonamides or 4-aminoquinolones in the urine<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
93