Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
(Continued)<br />
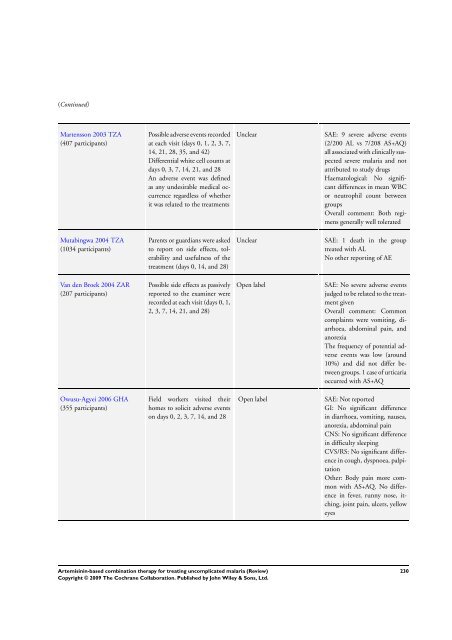
Martensson 2003 TZA<br />
(407 participants)<br />
Mutabingwa 2004 TZA<br />
(1034 participants)<br />
Van den Broek 2004 ZAR<br />
(207 participants)<br />
Owusu-Agyei 2006 GHA<br />
(355 participants)<br />
Possible adverse events recorded<br />
at each visit (days 0, 1, 2, 3, 7,<br />
14, 21, 28, 35, and 42)<br />
Differential white cell counts at<br />
days 0, 3, 7, 14, 21, and 28<br />
An adverse event was defined<br />
as any undesirable medical occurrence<br />
regardless of whether<br />
it was related to the treatments<br />
Parents or guardians were asked<br />
to report on side effects, tolerability<br />
and usefulness of the<br />
treatment (days 0, 14, and 28)<br />
Possible side effects as passively<br />
reported to the examiner were<br />
recorded at each visit (days 0, 1,<br />
2, 3, 7, 14, 21, and 28)<br />
Field workers visited their<br />
homes to solicit adverse events<br />
on days 0, 2, 3, 7, 14, and 28<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
Unclear SAE: 9 severe adverse events<br />
(2/200 AL vs 7/208 AS+AQ)<br />
all associated with clinically suspected<br />
severe malaria and not<br />
attributed to study drugs<br />
Haematological: No significant<br />
differences in mean WBC<br />
or neutrophil count between<br />
groups<br />
Overall comment: Both regimens<br />
generally well tolerated<br />
Unclear SAE: 1 death in the group<br />
treated with AL<br />
No other reporting of AE<br />
Open label SAE: No severe adverse events<br />
judged to be related to the treatment<br />
given<br />
Overall comment: Common<br />
complaints were vomiting, diarrhoea,<br />
abdominal pain, and<br />
anorexia<br />
<strong>The</strong> frequency of potential adverse<br />
events was low (around<br />
10%) and did not differ between<br />
groups. 1 case of urticaria<br />
occurred with AS+AQ<br />
Open label SAE: Not reported<br />
GI: No significant difference<br />
in diarrhoea, vomiting, nausea,<br />
anorexia, abdominal pain<br />
CNS: No significant difference<br />
in difficulty sleeping<br />
CVS/RS: No significant difference<br />
in cough, dyspnoea, palpitation<br />
Other: Body pain more common<br />
with AS+AQ. No difference<br />
in fever, runny nose, itching,<br />
joint pain, ulcers, yellow<br />
eyes<br />
230