Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
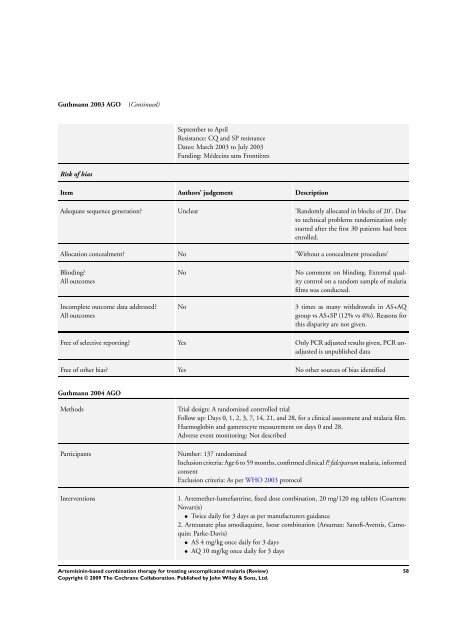
Guthmann 2003 AGO (Continued)<br />
Risk of bias<br />
September to April<br />
Resistance: CQ and SP resistance<br />
Dates: March 2003 to July 2003<br />
Funding: Médecins sans Frontières<br />
Item Authors’ judgement Description<br />
Adequate sequence generation? Unclear ’Randomly allocated in blocks of 20’. Due<br />
to technical problems randomization only<br />
started after the first 30 patients had been<br />
enrolled.<br />
Allocation concealment? No ’Without a concealment procedure’<br />
Blinding?<br />
All outcomes<br />
Incomplete outcome data addressed?<br />
All outcomes<br />
No No comment on blinding. External quality<br />
control on a random sample of malaria<br />
films was conducted.<br />
No 3 times as many withdrawals in AS+AQ<br />
group vs AS+SP (12% vs 4%). Reasons <strong>for</strong><br />
this disparity are not given.<br />
Free of selective reporting? Yes Only PCR adjusted results given, PCR unadjusted<br />
is unpublished data<br />
Free of other bias? Yes No other sources of bias identified<br />
Guthmann 2004 AGO<br />
Methods Trial design: A randomized controlled trial<br />
Follow up: Days 0, 1, 2, 3, 7, 14, 21, and 28, <strong>for</strong> a clinical assessment and malaria film.<br />
Haemoglobin and gametocyte measurement on days 0 and 28.<br />
Adverse event monitoring: Not described<br />
Participants Number: 137 randomized<br />
Inclusion criteria: Age 6 to 59 months, confirmed clinical P. falciparum malaria, in<strong>for</strong>med<br />
consent<br />
Exclusion criteria: As per WHO 2003 protocol<br />
Interventions 1. Artemether-lumefantrine, fixed dose <strong>combination</strong>, 20 mg/120 mg tablets (Coartem:<br />
Novartis)<br />
• Twice daily <strong>for</strong> 3 days as per manufacturers guidance<br />
2. Artesunate plus amodiaquine, loose <strong>combination</strong> (Arsumax: Sanofi-Aventis, Camoquin:<br />
Parke-Davis)<br />
• AS 4 mg/kg once daily <strong>for</strong> 3 days<br />
• AQ 10 mg/kg once daily <strong>for</strong> 3 days<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
58