Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
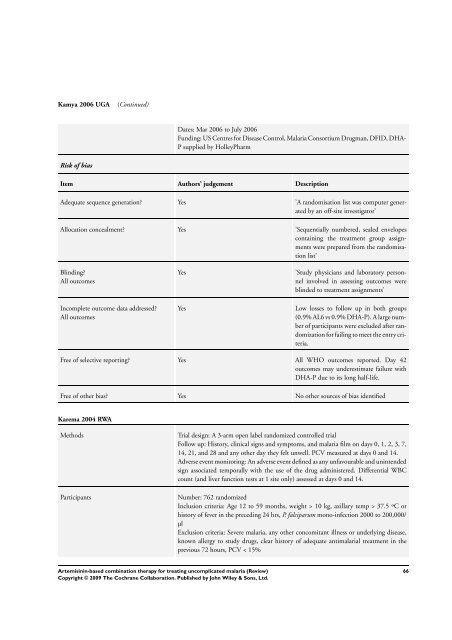
Kamya 2006 UGA (Continued)<br />
Risk of bias<br />
Dates: Mar 2006 to July 2006<br />
Funding: US Centres <strong>for</strong> Disease Control, Malaria Consortium Drugman, DFID, DHA-<br />
P supplied by HolleyPharm<br />
Item Authors’ judgement Description<br />
Adequate sequence generation? Yes ’A randomisation list was computer generated<br />
by an off-site investigator’<br />
Allocation concealment? Yes ’Sequentially numbered, sealed envelopes<br />
containing the treatment group assignments<br />
were prepared from the randomisation<br />
list’<br />
Blinding?<br />
All outcomes<br />
Incomplete outcome data addressed?<br />
All outcomes<br />
Yes ’Study physicians and laboratory personnel<br />
involved in assessing outcomes were<br />
blinded to treatment assignments’<br />
Yes Low losses to follow up in both groups<br />
(0.9% AL6 vs 0.9% DHA-P). A large number<br />
of participants were excluded after randomization<br />
<strong>for</strong> failing to meet the entry criteria.<br />
Free of selective reporting? Yes All WHO outcomes reported. Day 42<br />
outcomes may underestimate failure with<br />
DHA-P due to its long half-life.<br />
Free of other bias? Yes No other sources of bias identified<br />
Karema 2004 RWA<br />
Methods Trial design: A 3-arm open label randomized controlled trial<br />
Follow up: History, clinical signs and symptoms, and malaria film on days 0, 1, 2, 3, 7,<br />
14, 21, and 28 and any other day they felt unwell. PCV measured at days 0 and 14.<br />
Adverse event monitoring: An adverse event defined as any unfavourable and unintended<br />
sign associated temporally with the use of the drug administered. Differential WBC<br />
count (and liver function tests at 1 site only) assessed at days 0 and 14.<br />
Participants Number: 762 randomized<br />
Inclusion criteria: Age 12 to 59 months, weight > 10 kg, axillary temp > 37.5 ºC or<br />
history of fever in the preceding 24 hrs, P. falciparum mono-infection 2000 to 200,000/<br />
µl<br />
Exclusion criteria: Severe malaria, any other concomitant illness or underlying disease,<br />
known allergy to study drugs, clear history of adequate antimalarial treatment in the<br />
previous 72 hours, PCV < 15%<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
66