Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
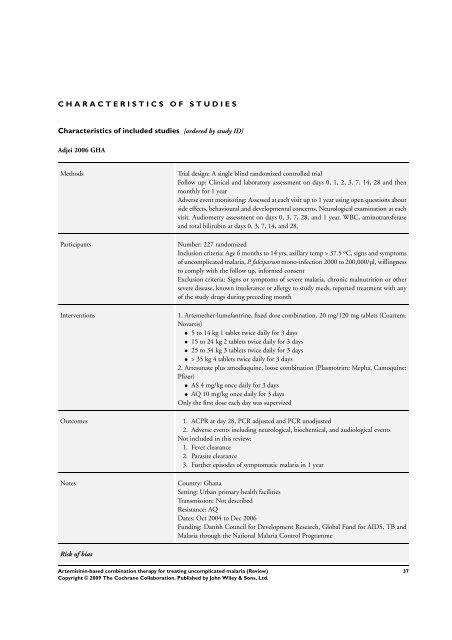
C H A R A C T E R I S T I C S O F S T U D I E S<br />
Characteristics of included studies [ordered by study ID]<br />
Adjei 2006 GHA<br />
Methods Trial design: A single blind randomized controlled trial<br />
Follow up: Clinical and laboratory assessment on days 0, 1, 2, 3, 7, 14, 28 and then<br />
monthly <strong>for</strong> 1 year<br />
Adverse event monitoring: Assessed at each visit up to 1 year using open questions about<br />
side effects, behavioural and developmental concerns. Neurological examination at each<br />
visit. Audiometry assessment on days 0, 3, 7, 28, and 1 year. WBC, aminotransferase<br />
and total bilirubin at days 0, 3, 7, 14, and 28.<br />
Participants Number: 227 randomized<br />
Inclusion criteria: Age 6 months to 14 yrs, axillary temp > 37.5 ºC, signs and symptoms<br />
of uncomplicated malaria, P. falciparum mono-infection 2000 to 200,000/µl, willingness<br />
to comply with the follow up, in<strong>for</strong>med consent<br />
Exclusion criteria: Signs or symptoms of severe malaria, chronic malnutrition or other<br />
severe disease, known intolerance or allergy to study meds, reported treatment with any<br />
of the study drugs during preceding month<br />
Interventions 1. Artemether-lumefantrine, fixed dose <strong>combination</strong>, 20 mg/120 mg tablets (Coartem:<br />
Novartis)<br />
• 5 to 14 kg 1 tablet twice daily <strong>for</strong> 3 days<br />
• 15 to 24 kg 2 tablets twice daily <strong>for</strong> 3 days<br />
• 25 to 34 kg 3 tablets twice daily <strong>for</strong> 3 days<br />
• > 35 kg 4 tablets twice daily <strong>for</strong> 3 days<br />
2. Artesunate plus amodiaquine, loose <strong>combination</strong> (Plasmotrim: Mepha, Camoquine:<br />
Pfizer)<br />
• AS 4 mg/kg once daily <strong>for</strong> 3 days<br />
• AQ 10 mg/kg once daily <strong>for</strong> 3 days<br />
Only the first dose each day was supervized<br />
Outcomes 1. ACPR at day 28, PCR adjusted and PCR unadjusted<br />
2. Adverse events including neurological, biochemical, and audiological events<br />
Not included in this review:<br />
1. Fever clearance<br />
2. Parasite clearance<br />
3. Further episodes of symptomatic malaria in 1 year<br />
Notes Country: Ghana<br />
Setting: Urban primary health facilities<br />
Transmission: Not described<br />
Resistance: AQ<br />
Dates: Oct 2004 to Dec 2006<br />
Funding: Danish Council <strong>for</strong> Development Research, Global Fund <strong>for</strong> AIDS, TB and<br />
Malaria through the National Malaria Control Programme<br />
Risk of bias<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
37