Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
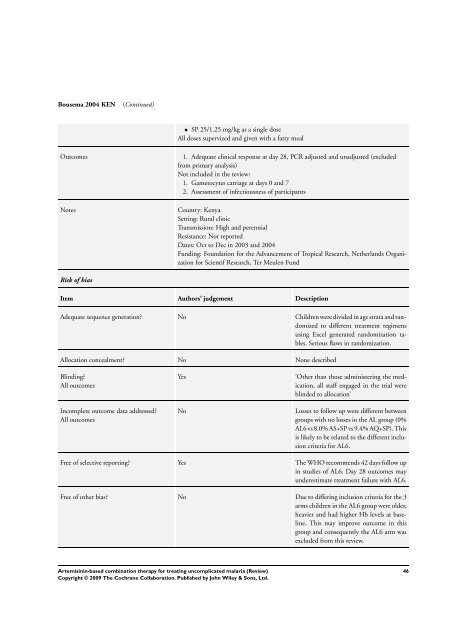
Bousema 2004 KEN (Continued)<br />
• SP 25/1.25 mg/kg as a single dose<br />
All doses supervized and given with a fatty meal<br />
Outcomes 1. Adequate clinical response at day 28, PCR adjusted and unadjusted (excluded<br />
from primary analysis)<br />
Not included in the review:<br />
1. Gametocytes carriage at days 0 and 7<br />
2. Assessment of infectiousness of participants<br />
Notes Country: Kenya<br />
Setting: Rural clinic<br />
Transmission: High and perennial<br />
Resistance: Not reported<br />
Dates: Oct to Dec in 2003 and 2004<br />
Funding: Foundation <strong>for</strong> the Advancement of Tropical Research, Netherlands Organization<br />
<strong>for</strong> Scientif Research, Ter Meulen Fund<br />
Risk of bias<br />
Item Authors’ judgement Description<br />
Adequate sequence generation? No Children were divided in age strata and randomized<br />
to different treatment regimens<br />
using Excel generated randomization tables.<br />
Serious flaws in randomization.<br />
Allocation concealment? No None described<br />
Blinding?<br />
All outcomes<br />
Incomplete outcome data addressed?<br />
All outcomes<br />
Yes ’Other than those administering the medication,<br />
all staff engaged in the trial were<br />
blinded to allocation’<br />
No Losses to follow up were different between<br />
groups with no losses in the AL group (0%<br />
AL6 vs 8.0% AS+SP vs 9.4% AQ+SP). This<br />
is likely to be related to the different inclusion<br />
criteria <strong>for</strong> AL6.<br />
Free of selective reporting? Yes <strong>The</strong> WHO recommends 42 days follow up<br />
in studies of AL6. Day 28 outcomes may<br />
underestimate treatment failure with AL6.<br />
Free of other bias? No Due to differing inclusion criteria <strong>for</strong> the 3<br />
arms children in the AL6 group were older,<br />
heavier and had higher Hb levels at baseline.<br />
This may improve outcome in this<br />
group and consequently the AL6 arm was<br />
excluded from this review.<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
46