Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
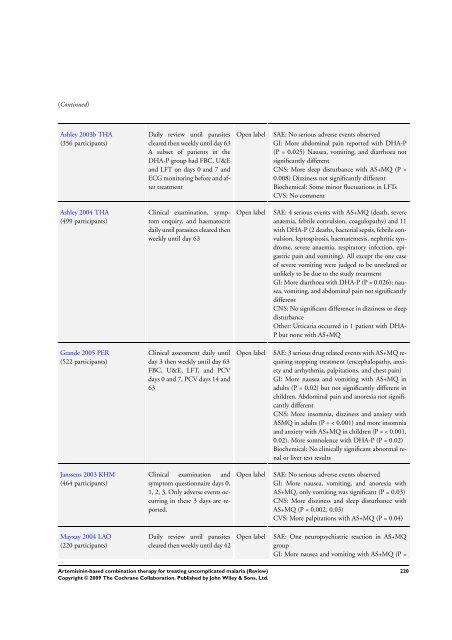
(Continued)<br />
Ashley 2003b THA<br />
(356 participants)<br />
Ashley 2004 THA<br />
(499 participants)<br />
Grande 2005 PER<br />
(522 participants)<br />
Janssens 2003 KHM<br />
(464 participants)<br />
Mayxay 2004 LAO<br />
(220 participants)<br />
Daily review until parasites<br />
cleared then weekly until day 63<br />
A subset of patients in the<br />
DHA-P group had FBC, U&E<br />
and LFT on days 0 and 7 and<br />
ECG monitoring be<strong>for</strong>e and after<br />
treatment<br />
Clinical examination, symptom<br />
enquiry, and haematocrit<br />
daily until parasites cleared then<br />
weekly until day 63<br />
Clinical assessment daily until<br />
day 3 then weekly until day 63<br />
FBC, U&E, LFT, and PCV<br />
days 0 and 7, PCV days 14 and<br />
63<br />
Clinical examination and<br />
symptom questionnaire days 0,<br />
1, 2, 3. Only adverse events occurring<br />
in these 3 days are reported.<br />
Daily review until parasites<br />
cleared then weekly until day 42<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
Open label SAE: No serious adverse events observed<br />
GI: More abdominal pain reported with DHA-P<br />
(P = 0.025) Nausea, vomiting, and diarrhoea not<br />
significantly different<br />
CNS: More sleep disturbance with AS+MQ (P =<br />
0.008) Dizziness not significantly different<br />
Biochemical: Some minor fluctuations in LFTs<br />
CVS: No comment<br />
Open label SAE: 4 serious events with AS+MQ (death, severe<br />
anaemia, febrile convulsion, coagulopathy) and 11<br />
with DHA-P (2 deaths, bacterial sepsis, febrile convulsion,<br />
leptospirosis, haematemesis, nephritic syndrome,<br />
severe anaemia, respiratory infection, epigastric<br />
pain and vomiting). All except the one case<br />
of severe vomiting were judged to be unrelated or<br />
unlikely to be due to the study treatment<br />
GI: More diarrhoea with DHA-P (P = 0.026); nausea,<br />
vomiting, and abdominal pain not significantly<br />
different<br />
CNS: No significant difference in dizziness or sleep<br />
disturbance<br />
Other: Urticaria occurred in 1 patient with DHA-<br />
P but none with AS+MQ<br />
Open label SAE: 3 serious drug related events with AS+MQ requiring<br />
stopping treatment (encephalopathy, anxiety<br />
and arrhythmia, palpitations, and chest pain)<br />
GI: More nausea and vomiting with AS+MQ in<br />
adults (P = 0.02) but not significantly different in<br />
children. Abdominal pain and anorexia not significantly<br />
different<br />
CNS: More insomnia, dizziness and anxiety with<br />
ASMQ in adults (P = < 0.001) and more insomnia<br />
and anxiety with AS+MQ in children (P = < 0.001,<br />
0.02). More somnolence with DHA-P (P = 0.02)<br />
Biochemical: No clinically significant abnormal renal<br />
or liver test results<br />
Open label SAE: No serious adverse events observed<br />
GI: More nausea, vomiting, and anorexia with<br />
AS+MQ, only vomiting was significant (P = 0.03)<br />
CNS: More dizziness and sleep disturbance with<br />
AS+MQ (P = 0.002, 0.03)<br />
CVS: More palpitations with AS+MQ (P = 0.04)<br />
Open label SAE: One neuropsychiatric reaction in AS+MQ<br />
group<br />
GI: More nausea and vomiting with AS+MQ (P =<br />
220