Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
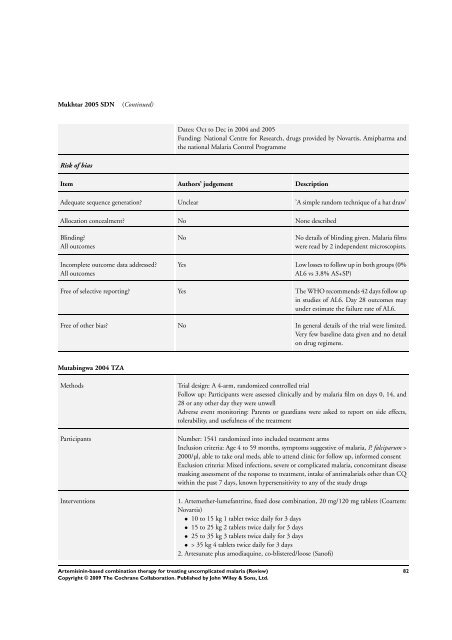
Mukhtar 2005 SDN (Continued)<br />
Risk of bias<br />
Dates: Oct to Dec in 2004 and 2005<br />
Funding: National Centre <strong>for</strong> Research, drugs provided by Novartis, Amipharma and<br />
the national Malaria Control Programme<br />
Item Authors’ judgement Description<br />
Adequate sequence generation? Unclear ’A simple random technique of a hat draw’<br />
Allocation concealment? No None described<br />
Blinding?<br />
All outcomes<br />
Incomplete outcome data addressed?<br />
All outcomes<br />
No No details of blinding given. Malaria films<br />
were read by 2 independent microscopists.<br />
Yes Low losses to follow up in both groups (0%<br />
AL6 vs 3.8% AS+SP)<br />
Free of selective reporting? Yes <strong>The</strong> WHO recommends 42 days follow up<br />
in studies of AL6. Day 28 outcomes may<br />
under estimate the failure rate of AL6.<br />
Free of other bias? No In general details of the trial were limited.<br />
Very few baseline data given and no detail<br />
on drug regimens.<br />
Mutabingwa 2004 TZA<br />
Methods Trial design: A 4-arm, randomized controlled trial<br />
Follow up: Participants were assessed clinically and by malaria film on days 0, 14, and<br />
28 or any other day they were unwell<br />
Adverse event monitoring: Parents or guardians were asked to report on side effects,<br />
tolerability, and usefulness of the treatment<br />
Participants Number: 1541 randomized into included treatment arms<br />
Inclusion criteria: Age 4 to 59 months, symptoms suggestive of malaria, P. falciparum ><br />
2000/µl, able to take oral meds, able to attend clinic <strong>for</strong> follow up, in<strong>for</strong>med consent<br />
Exclusion criteria: Mixed infections, severe or complicated malaria, concomitant disease<br />
masking assessment of the response to treatment, intake of antimalarials other than CQ<br />
within the past 7 days, known hypersensitivity to any of the study drugs<br />
Interventions 1. Artemether-lumefantrine, fixed dose <strong>combination</strong>, 20 mg/120 mg tablets (Coartem:<br />
Novartis)<br />
• 10 to 15 kg 1 tablet twice daily <strong>for</strong> 3 days<br />
• 15 to 25 kg 2 tablets twice daily <strong>for</strong> 3 days<br />
• 25 to 35 kg 3 tablets twice daily <strong>for</strong> 3 days<br />
• > 35 kg 4 tablets twice daily <strong>for</strong> 3 days<br />
2. Artesunate plus amodiaquine, co-blistered/loose (Sanofi)<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
82