Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
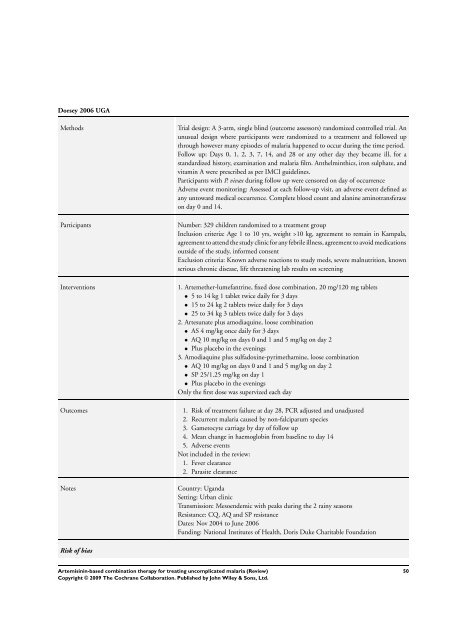
Dorsey 2006 UGA<br />
Methods Trial design: A 3-arm, single blind (outcome assessors) randomized controlled trial. An<br />
unusual design where participants were randomized to a treatment and followed up<br />
through however many episodes of malaria happened to occur during the time period.<br />
Follow up: Days 0, 1, 2, 3, 7, 14, and 28 or any other day they became ill, <strong>for</strong> a<br />
standardized history, examination and malaria film. Anthelminthics, iron sulphate, and<br />
vitamin A were prescribed as per IMCI guidelines.<br />
Participants with P. vivax during follow up were censored on day of occurrence<br />
Adverse event monitoring: Assessed at each follow-up visit, an adverse event defined as<br />
any untoward medical occurrence. Complete blood count and alanine aminotransferase<br />
on day 0 and 14.<br />
Participants Number: 329 children randomized to a treatment group<br />
Inclusion criteria: Age 1 to 10 yrs, weight >10 kg, agreement to remain in Kampala,<br />
agreement to attend the study clinic <strong>for</strong> any febrile illness, agreement to avoid medications<br />
outside of the study, in<strong>for</strong>med consent<br />
Exclusion criteria: Known adverse reactions to study meds, severe malnutrition, known<br />
serious chronic disease, life threatening lab results on screening<br />
Interventions 1. Artemether-lumefantrine, fixed dose <strong>combination</strong>, 20 mg/120 mg tablets<br />
• 5 to 14 kg 1 tablet twice daily <strong>for</strong> 3 days<br />
• 15 to 24 kg 2 tablets twice daily <strong>for</strong> 3 days<br />
• 25 to 34 kg 3 tablets twice daily <strong>for</strong> 3 days<br />
2. Artesunate plus amodiaquine, loose <strong>combination</strong><br />
• AS 4 mg/kg once daily <strong>for</strong> 3 days<br />
• AQ 10 mg/kg on days 0 and 1 and 5 mg/kg on day 2<br />
• Plus placebo in the evenings<br />
3. Amodiaquine plus sulfadoxine-pyrimethamine, loose <strong>combination</strong><br />
• AQ 10 mg/kg on days 0 and 1 and 5 mg/kg on day 2<br />
• SP 25/1.25 mg/kg on day 1<br />
• Plus placebo in the evenings<br />
Only the first dose was supervized each day<br />
Outcomes 1. Risk of treatment failure at day 28, PCR adjusted and unadjusted<br />
2. Recurrent malaria caused by non-falciparum species<br />
3. Gametocyte carriage by day of follow up<br />
4. Mean change in haemoglobin from baseline to day 14<br />
5. Adverse events<br />
Not included in the review:<br />
1. Fever clearance<br />
2. Parasite clearance<br />
Notes Country: Uganda<br />
Setting: Urban clinic<br />
Transmission: Mesoendemic with peaks during the 2 rainy seasons<br />
Resistance: CQ, AQ and SP resistance<br />
Dates: Nov 2004 to June 2006<br />
Funding: National Institutes of Health, Doris Duke Charitable Foundation<br />
Risk of bias<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
50