Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
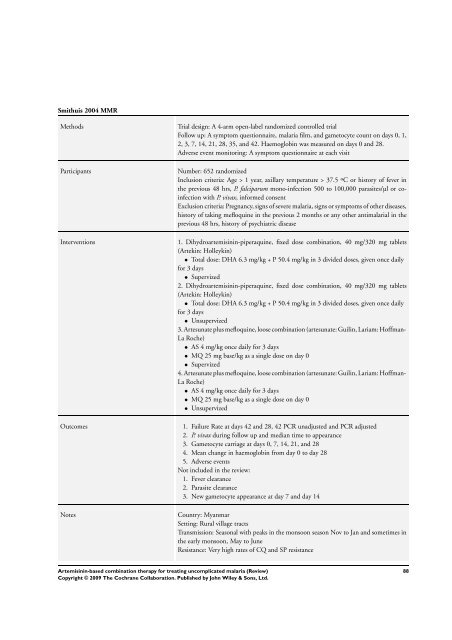
Smithuis 2004 MMR<br />
Methods Trial design: A 4-arm open-label randomized controlled trial<br />
Follow up: A symptom questionnaire, malaria film, and gametocyte count on days 0, 1,<br />
2, 3, 7, 14, 21, 28, 35, and 42. Haemoglobin was measured on days 0 and 28.<br />
Adverse event monitoring: A symptom questionnaire at each visit<br />
Participants Number: 652 randomized<br />
Inclusion criteria: Age > 1 year, axillary temperature > 37.5 ºC or history of fever in<br />
the previous 48 hrs, P. falciparum mono-infection 500 to 100,000 parasites/µl or coinfection<br />
with P. vivax, in<strong>for</strong>med consent<br />
Exclusion criteria: Pregnancy, signs of severe malaria, signs or symptoms of other diseases,<br />
history of taking mefloquine in the previous 2 months or any other antimalarial in the<br />
previous 48 hrs, history of psychiatric disease<br />
Interventions 1. Dihydroartemisinin-piperaquine, fixed dose <strong>combination</strong>, 40 mg/320 mg tablets<br />
(Artekin: Holleykin)<br />
• Total dose: DHA 6.3 mg/kg + P 50.4 mg/kg in 3 divided doses, given once daily<br />
<strong>for</strong> 3 days<br />
• Supervized<br />
2. Dihydroartemisinin-piperaquine, fixed dose <strong>combination</strong>, 40 mg/320 mg tablets<br />
(Artekin: Holleykin)<br />
• Total dose: DHA 6.3 mg/kg + P 50.4 mg/kg in 3 divided doses, given once daily<br />
<strong>for</strong> 3 days<br />
• Unsupervized<br />
3. Artesunate plus mefloquine, loose <strong>combination</strong> (artesunate: Guilin, Lariam: Hoffman-<br />
La Roche)<br />
• AS 4 mg/kg once daily <strong>for</strong> 3 days<br />
• MQ 25 mg base/kg as a single dose on day 0<br />
• Supervized<br />
4. Artesunate plus mefloquine, loose <strong>combination</strong> (artesunate: Guilin, Lariam: Hoffman-<br />
La Roche)<br />
• AS 4 mg/kg once daily <strong>for</strong> 3 days<br />
• MQ 25 mg base/kg as a single dose on day 0<br />
• Unsupervized<br />
Outcomes 1. Failure Rate at days 42 and 28, 42 PCR unadjusted and PCR adjusted<br />
2. P. vivax during follow up and median time to appearance<br />
3. Gametocyte carriage at days 0, 7, 14, 21, and 28<br />
4. Mean change in haemoglobin from day 0 to day 28<br />
5. Adverse events<br />
Not included in the review:<br />
1. Fever clearance<br />
2. Parasite clearance<br />
3. New gametocyte appearance at day 7 and day 14<br />
Notes Country: Myanmar<br />
Setting: Rural village tracts<br />
Transmission: Seasonal with peaks in the monsoon season Nov to Jan and sometimes in<br />
the early monsoon, May to June<br />
Resistance: Very high rates of CQ and SP resistance<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
88