Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
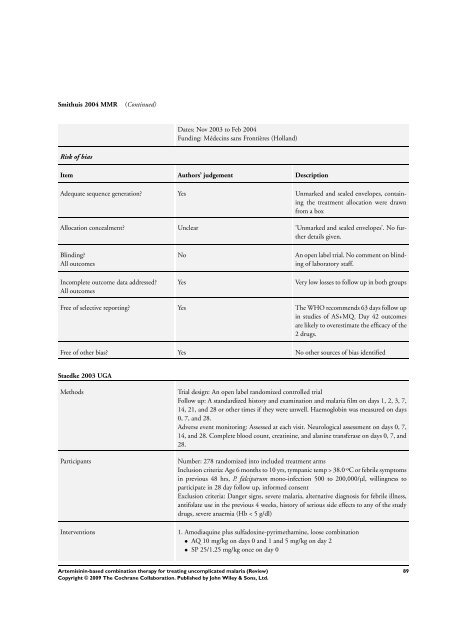
Smithuis 2004 MMR (Continued)<br />
Risk of bias<br />
Dates: Nov 2003 to Feb 2004<br />
Funding: Médecins sans Frontières (Holland)<br />
Item Authors’ judgement Description<br />
Adequate sequence generation? Yes Unmarked and sealed envelopes, containing<br />
the treatment allocation were drawn<br />
from a box<br />
Allocation concealment? Unclear ’Unmarked and sealed envelopes’. No further<br />
details given.<br />
Blinding?<br />
All outcomes<br />
Incomplete outcome data addressed?<br />
All outcomes<br />
No An open label trial. No comment on blinding<br />
of laboratory staff.<br />
Yes Very low losses to follow up in both groups<br />
Free of selective reporting? Yes <strong>The</strong> WHO recommends 63 days follow up<br />
in studies of AS+MQ. Day 42 outcomes<br />
are likely to overestimate the efficacy of the<br />
2 drugs.<br />
Free of other bias? Yes No other sources of bias identified<br />
Staedke 2003 UGA<br />
Methods Trial design: An open label randomized controlled trial<br />
Follow up: A standardized history and examination and malaria film on days 1, 2, 3, 7,<br />
14, 21, and 28 or other times if they were unwell. Haemoglobin was measured on days<br />
0, 7, and 28.<br />
Adverse event monitoring: Assessed at each visit. Neurological assessment on days 0, 7,<br />
14, and 28. Complete blood count, creatinine, and alanine transferase on days 0, 7, and<br />
28.<br />
Participants Number: 278 randomized into included treatment arms<br />
Inclusion criteria: Age 6 months to 10 yrs, tympanic temp > 38.0 ºC or febrile symptoms<br />
in previous 48 hrs, P. falciparum mono-infection 500 to 200,000/µl, willingness to<br />
participate in 28 day follow up, in<strong>for</strong>med consent<br />
Exclusion criteria: Danger signs, severe malaria, alternative diagnosis <strong>for</strong> febrile illness,<br />
antifolate use in the previous 4 weeks, history of serious side effects to any of the study<br />
drugs, severe anaemia (Hb < 5 g/dl)<br />
Interventions 1. Amodiaquine plus sulfadoxine-pyrimethamine, loose <strong>combination</strong><br />
• AQ 10 mg/kg on days 0 and 1 and 5 mg/kg on day 2<br />
• SP 25/1.25 mg/kg once on day 0<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
89