Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
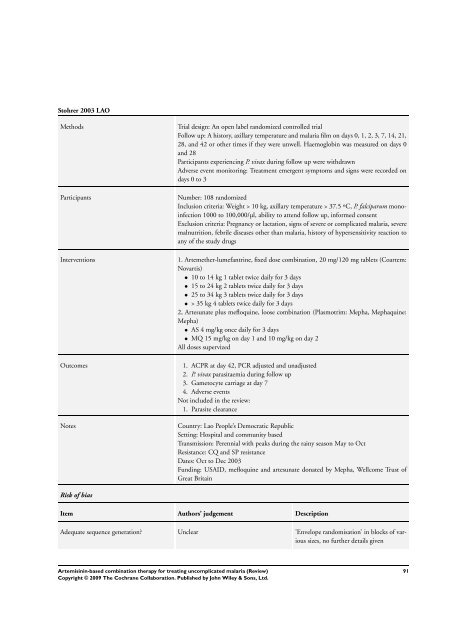
Stohrer 2003 LAO<br />
Methods Trial design: An open label randomized controlled trial<br />
Follow up: A history, axillary temperature and malaria film on days 0, 1, 2, 3, 7, 14, 21,<br />
28, and 42 or other times if they were unwell. Haemoglobin was measured on days 0<br />
and 28<br />
Participants experiencing P. vivax during follow up were withdrawn<br />
Adverse event monitoring: Treatment emergent symptoms and signs were recorded on<br />
days 0 to 3<br />
Participants Number: 108 randomized<br />
Inclusion criteria: Weight > 10 kg, axillary temperature > 37.5 ºC, P. falciparum monoinfection<br />
1000 to 100,000/µl, ability to attend follow up, in<strong>for</strong>med consent<br />
Exclusion criteria: Pregnancy or lactation, signs of severe or complicated malaria, severe<br />
malnutrition, febrile diseases other than malaria, history of hypersensitivity reaction to<br />
any of the study drugs<br />
Interventions 1. Artemether-lumefantrine, fixed dose <strong>combination</strong>, 20 mg/120 mg tablets (Coartem:<br />
Novartis)<br />
• 10 to 14 kg 1 tablet twice daily <strong>for</strong> 3 days<br />
• 15 to 24 kg 2 tablets twice daily <strong>for</strong> 3 days<br />
• 25 to 34 kg 3 tablets twice daily <strong>for</strong> 3 days<br />
• > 35 kg 4 tablets twice daily <strong>for</strong> 3 days<br />
2, Artesunate plus mefloquine, loose <strong>combination</strong> (Plasmotrim: Mepha, Mephaquine:<br />
Mepha)<br />
• AS 4 mg/kg once daily <strong>for</strong> 3 days<br />
• MQ 15 mg/kg on day 1 and 10 mg/kg on day 2<br />
All doses supervized<br />
Outcomes 1. ACPR at day 42, PCR adjusted and unadjusted<br />
2. P. vivax parasitaemia during follow up<br />
3. Gametocyte carriage at day 7<br />
4. Adverse events<br />
Not included in the review:<br />
1. Parasite clearance<br />
Notes Country: Lao People’s Democratic Republic<br />
Setting: Hospital and community <strong>based</strong><br />
Transmission: Perennial with peaks during the rainy season May to Oct<br />
Resistance: CQ and SP resistance<br />
Dates: Oct to Dec 2003<br />
Funding: USAID, mefloquine and artesunate donated by Mepha, Wellcome Trust of<br />
Great Britain<br />
Risk of bias<br />
Item Authors’ judgement Description<br />
Adequate sequence generation? Unclear ’Envelope randomisation’ in blocks of various<br />
sizes, no further details given<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
91