Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
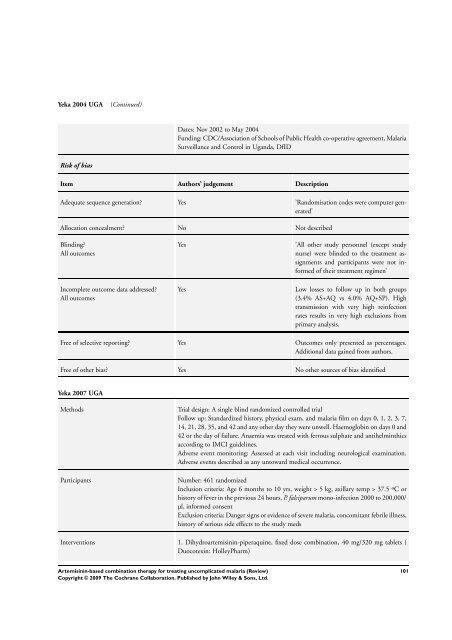
Yeka 2004 UGA (Continued)<br />
Risk of bias<br />
Dates: Nov 2002 to May 2004<br />
Funding: CDC/Association of Schools of Public Health co-operative agreement, Malaria<br />
Surveillance and Control in Uganda, DfID<br />
Item Authors’ judgement Description<br />
Adequate sequence generation? Yes ’Randomisation codes were computer generated’<br />
Allocation concealment? No Not described<br />
Blinding?<br />
All outcomes<br />
Incomplete outcome data addressed?<br />
All outcomes<br />
Yes ’All other study personnel (except study<br />
nurse) were blinded to the treatment assignments<br />
and participants were not in<strong>for</strong>med<br />
of their treatment regimen’<br />
Yes Low losses to follow up in both groups<br />
(3.4% AS+AQ vs 4.0% AQ+SP). High<br />
transmission with very high reinfection<br />
rates results in very high exclusions from<br />
primary analysis.<br />
Free of selective reporting? Yes Outcomes only presented as percentages.<br />
Additional data gained from authors.<br />
Free of other bias? Yes No other sources of bias identified<br />
Yeka 2007 UGA<br />
Methods Trial design: A single blind randomized controlled trial<br />
Follow up: Standardized history, physical exam, and malaria film on days 0, 1, 2, 3, 7,<br />
14, 21, 28, 35, and 42 and any other day they were unwell. Haemoglobin on days 0 and<br />
42 or the day of failure. Anaemia was treated with ferrous sulphate and antihelminthics<br />
according to IMCI guidelines.<br />
Adverse event monitoring: Assessed at each visit including neurological examination.<br />
Adverse events described as any untoward medical occurrence.<br />
Participants Number: 461 randomized<br />
Inclusion criteria: Age 6 months to 10 yrs, weight > 5 kg, axillary temp > 37.5 ºC or<br />
history of fever in the previous 24 hours, P. falciparum mono-infection 2000 to 200,000/<br />
µl, in<strong>for</strong>med consent<br />
Exclusion criteria: Danger signs or evidence of severe malaria, concomitant febrile illness,<br />
history of serious side effects to the study meds<br />
Interventions 1. Dihydroartemisinin-piperaquine, fixed dose <strong>combination</strong>, 40 mg/320 mg tablets (<br />
Duocotexin: HolleyPharm)<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
101