Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
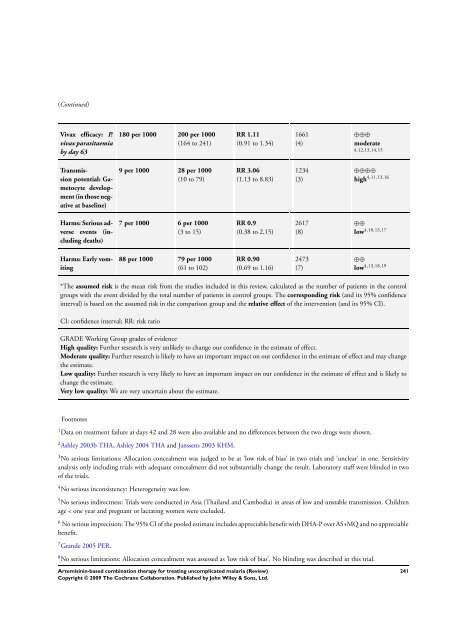
(Continued)<br />
Vivax efficacy: P.<br />
vivax parasitaemia<br />
by day 63<br />
Transmission<br />
potential: Gametocytedevelopment<br />
(in those negative<br />
at baseline)<br />
Harms: Serious adverse<br />
events (including<br />
deaths)<br />
Harms: Early vomiting<br />
180 per 1000 200 per 1000<br />
(164 to 241)<br />
9 per 1000 28 per 1000<br />
(10 to 79)<br />
7 per 1000 6 per 1000<br />
(3 to 15)<br />
88 per 1000 79 per 1000<br />
(61 to 102)<br />
RR 1.11<br />
(0.91 to 1.34)<br />
RR 3.06<br />
(1.13 to 8.83)<br />
RR 0.9<br />
(0.38 to 2.15)<br />
RR 0.90<br />
(0.69 to 1.16)<br />
1661<br />
(4)<br />
1234<br />
(3)<br />
2617<br />
(8)<br />
2473<br />
(7)<br />
⊕⊕⊕<br />
moderate<br />
4,12,13,14,15<br />
⊕⊕⊕⊕<br />
high 4,11,13,16<br />
⊕⊕<br />
low 4,10,13,17<br />
⊕⊕<br />
low 4,13,18,19<br />
*<strong>The</strong> assumed risk is the mean risk from the studies included in this review, calculated as the number of patients in the control<br />
groups with the event divided by the total number of patients in control groups. <strong>The</strong> corresponding risk (and its 95% confidence<br />
interval) is <strong>based</strong> on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).<br />
CI: confidence interval; RR: risk ratio<br />
GRADE Working Group grades of evidence<br />
High quality: Further research is very unlikely to change our confidence in the estimate of effect.<br />
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change<br />
the estimate.<br />
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to<br />
change the estimate.<br />
Very low quality: We are very uncertain about the estimate.<br />
Footnotes<br />
1 Data on treatment failure at days 42 and 28 were also available and no differences between the two drugs were shown.<br />
2 Ashley 2003b THA, Ashley 2004 THA and Janssens 2003 KHM.<br />
3 No serious limitations: Allocation concealment was judged to be at ’low risk of bias’ in two trials and ’unclear’ in one. Sensitivity<br />
analysis only including trials with adequate concealment did not substantially change the result. Laboratory staff were blinded in two<br />
of the trials.<br />
4 No serious inconsistency: Heterogeneity was low.<br />
5 No serious indirectness: Trials were conducted in Asia (Thailand and Cambodia) in areas of low and unstable transmission. Children<br />
age < one year and pregnant or lactating women were excluded.<br />
6 No serious imprecision: <strong>The</strong> 95% CI of the pooled estimate includes appreciable benefit with DHA-P over AS+MQ and no appreciable<br />
benefit.<br />
7 Grande 2005 PER.<br />
8 No serious limitations: Allocation concealment was assessed as ’low risk of bias’. No blinding was described in this trial.<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
241