Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
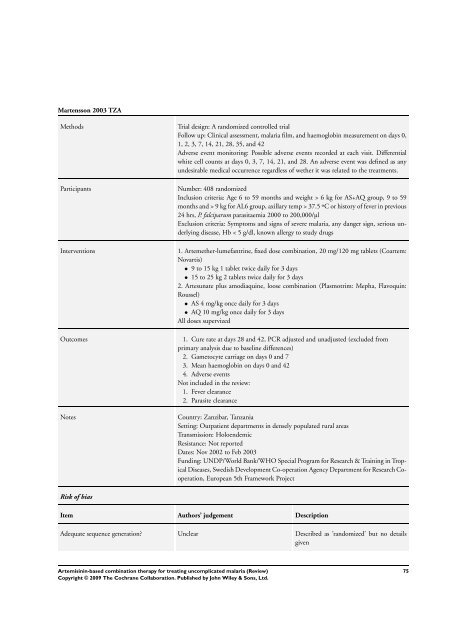
Martensson 2003 TZA<br />
Methods Trial design: A randomized controlled trial<br />
Follow up: Clinical assessment, malaria film, and haemoglobin measurement on days 0,<br />
1, 2, 3, 7, 14, 21, 28, 35, and 42<br />
Adverse event monitoring: Possible adverse events recorded at each visit. Differential<br />
white cell counts at days 0, 3, 7, 14, 21, and 28. An adverse event was defined as any<br />
undesirable medical occurrence regardless of wether it was related to the treatments.<br />
Participants Number: 408 randomized<br />
Inclusion criteria: Age 6 to 59 months and weight > 6 kg <strong>for</strong> AS+AQ group, 9 to 59<br />
months and > 9 kg <strong>for</strong> AL6 group, axillary temp > 37.5 ºC or history of fever in previous<br />
24 hrs, P. falciparum parasitaemia 2000 to 200,000/µl<br />
Exclusion criteria: Symptoms and signs of severe malaria, any danger sign, serious underlying<br />
disease, Hb < 5 g/dl, known allergy to study drugs<br />
Interventions 1. Artemether-lumefantrine, fixed dose <strong>combination</strong>, 20 mg/120 mg tablets (Coartem:<br />
Novartis)<br />
• 9 to 15 kg 1 tablet twice daily <strong>for</strong> 3 days<br />
• 15 to 25 kg 2 tablets twice daily <strong>for</strong> 3 days<br />
2. Artesunate plus amodiaquine, loose <strong>combination</strong> (Plasmotrim: Mepha, Flavoquin:<br />
Roussel)<br />
• AS 4 mg/kg once daily <strong>for</strong> 3 days<br />
• AQ 10 mg/kg once daily <strong>for</strong> 3 days<br />
All doses supervized<br />
Outcomes 1. Cure rate at days 28 and 42, PCR adjusted and unadjusted (excluded from<br />
primary analysis due to baseline differences)<br />
2. Gametocyte carriage on days 0 and 7<br />
3. Mean haemoglobin on days 0 and 42<br />
4. Adverse events<br />
Not included in the review:<br />
1. Fever clearance<br />
2. Parasite clearance<br />
Notes Country: Zanzibar, Tanzania<br />
Setting: Outpatient departments in densely populated rural areas<br />
Transmission: Holoendemic<br />
Resistance: Not reported<br />
Dates: Nov 2002 to Feb 2003<br />
Funding: UNDP/World Bank/WHO Special Program <strong>for</strong> Research & Training in Tropical<br />
Diseases, Swedish Development Co-operation Agency Department <strong>for</strong> Research Cooperation,<br />
European 5th Framework Project<br />
Risk of bias<br />
Item Authors’ judgement Description<br />
Adequate sequence generation? Unclear Described as ’randomized’ but no details<br />
given<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
75