Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
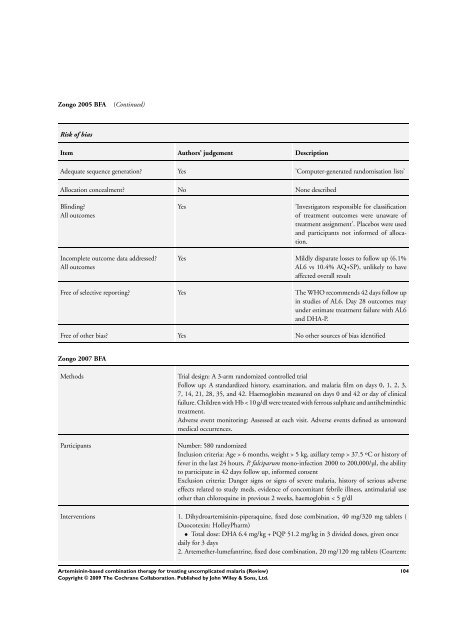
Zongo 2005 BFA (Continued)<br />
Risk of bias<br />
Item Authors’ judgement Description<br />
Adequate sequence generation? Yes ’Computer-generated randomisation lists’<br />
Allocation concealment? No None described<br />
Blinding?<br />
All outcomes<br />
Incomplete outcome data addressed?<br />
All outcomes<br />
Yes ’Investigators responsible <strong>for</strong> classification<br />
of treatment outcomes were unaware of<br />
treatment assignment’. Placebos were used<br />
and participants not in<strong>for</strong>med of allocation.<br />
Yes Mildly disparate losses to follow up (6.1%<br />
AL6 vs 10.4% AQ+SP), unlikely to have<br />
affected overall result<br />
Free of selective reporting? Yes <strong>The</strong> WHO recommends 42 days follow up<br />
in studies of AL6. Day 28 outcomes may<br />
under estimate treatment failure with AL6<br />
and DHA-P.<br />
Free of other bias? Yes No other sources of bias identified<br />
Zongo 2007 BFA<br />
Methods Trial design: A 3-arm randomized controlled trial<br />
Follow up: A standardized history, examination, and malaria film on days 0, 1, 2, 3,<br />
7, 14, 21, 28, 35, and 42. Haemoglobin measured on days 0 and 42 or day of clinical<br />
failure. Children with Hb < 10 g/dl were treated with ferrous sulphate and antihelminthic<br />
treatment.<br />
Adverse event monitoring: Assessed at each visit. Adverse events defined as untoward<br />
medical occurrences.<br />
Participants Number: 580 randomized<br />
Inclusion criteria: Age > 6 months, weight > 5 kg, axillary temp > 37.5 ºC or history of<br />
fever in the last 24 hours, P. falciparum mono-infection 2000 to 200,000/µl, the ability<br />
to participate in 42 days follow up, in<strong>for</strong>med consent<br />
Exclusion criteria: Danger signs or signs of severe malaria, history of serious adverse<br />
effects related to study meds, evidence of concomitant febrile illness, antimalarial use<br />
other than chloroquine in previous 2 weeks, haemoglobin < 5 g/dl<br />
Interventions 1. Dihydroartemisinin-piperaquine, fixed dose <strong>combination</strong>, 40 mg/320 mg tablets (<br />
Duocotexin: HolleyPharm)<br />
• Total dose: DHA 6.4 mg/kg + PQP 51.2 mg/kg in 3 divided doses, given once<br />
daily <strong>for</strong> 3 days<br />
2. Artemether-lumefantrine, fixed dose <strong>combination</strong>, 20 mg/120 mg tablets (Coartem:<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
104