Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
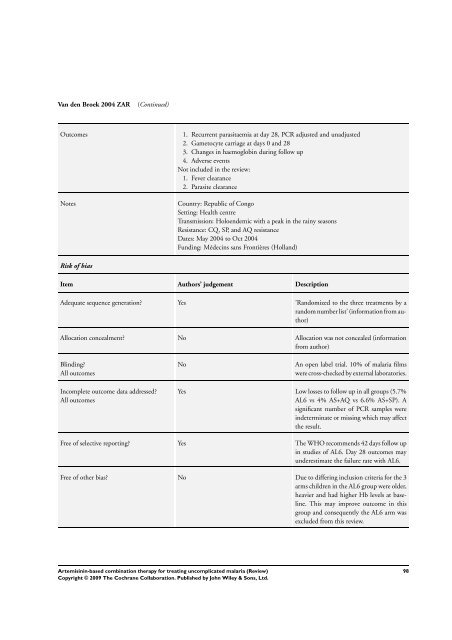
Van den Broek 2004 ZAR (Continued)<br />
Outcomes 1. Recurrent parasitaemia at day 28, PCR adjusted and unadjusted<br />
2. Gametocyte carriage at days 0 and 28<br />
3. Changes in haemoglobin during follow up<br />
4. Adverse events<br />
Not included in the review:<br />
1. Fever clearance<br />
2. Parasite clearance<br />
Notes Country: Republic of Congo<br />
Setting: Health centre<br />
Transmission: Holoendemic with a peak in the rainy seasons<br />
Resistance: CQ, SP, and AQ resistance<br />
Dates: May 2004 to Oct 2004<br />
Funding: Médecins sans Frontières (Holland)<br />
Risk of bias<br />
Item Authors’ judgement Description<br />
Adequate sequence generation? Yes ’Randomized to the three treatments by a<br />
random number list’ (in<strong>for</strong>mation from author)<br />
Allocation concealment? No Allocation was not concealed (in<strong>for</strong>mation<br />
from author)<br />
Blinding?<br />
All outcomes<br />
Incomplete outcome data addressed?<br />
All outcomes<br />
No An open label trial. 10% of malaria films<br />
were cross-checked by external laboratories.<br />
Yes Low losses to follow up in all groups (5.7%<br />
AL6 vs 4% AS+AQ vs 6.6% AS+SP). A<br />
significant number of PCR samples were<br />
indeterminate or missing which may affect<br />
the result.<br />
Free of selective reporting? Yes <strong>The</strong> WHO recommends 42 days follow up<br />
in studies of AL6. Day 28 outcomes may<br />
underestimate the failure rate with AL6.<br />
Free of other bias? No Due to differing inclusion criteria <strong>for</strong> the 3<br />
arms children in the AL6 group were older,<br />
heavier and had higher Hb levels at baseline.<br />
This may improve outcome in this<br />
group and consequently the AL6 arm was<br />
excluded from this review.<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
98