Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
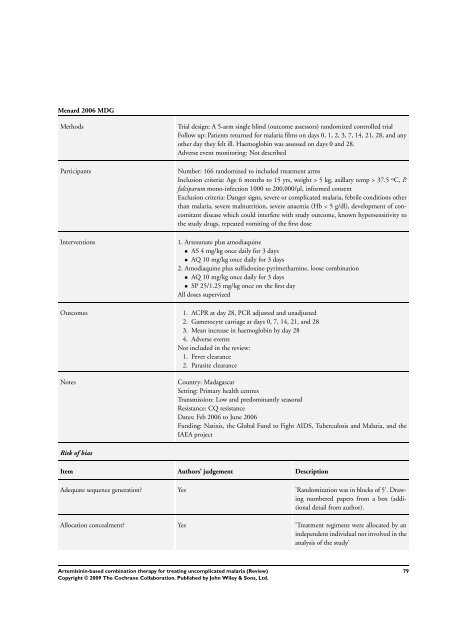
Menard 2006 MDG<br />
Methods Trial design: A 5-arm single blind (outcome assessors) randomized controlled trial<br />
Follow up: Patients returned <strong>for</strong> malaria films on days 0, 1, 2, 3, 7, 14, 21, 28, and any<br />
other day they felt ill. Haemoglobin was assessed on days 0 and 28.<br />
Adverse event monitoring: Not described<br />
Participants Number: 166 randomized to included treatment arms<br />
Inclusion criteria: Age 6 months to 15 yrs, weight > 5 kg, axillary temp > 37.5 ºC, P.<br />
falciparum mono-infection 1000 to 200,000/µl, in<strong>for</strong>med consent<br />
Exclusion criteria: Danger signs, severe or complicated malaria, febrile conditions other<br />
than malaria, severe malnutrition, severe anaemia (Hb < 5 g/dl), development of concomitant<br />
disease which could interfere with study outcome, known hypersensitivity to<br />
the study drugs, repeated vomiting of the first dose<br />
Interventions 1. Artesunate plus amodiaquine<br />
• AS 4 mg/kg once daily <strong>for</strong> 3 days<br />
• AQ 10 mg/kg once daily <strong>for</strong> 3 days<br />
2. Amodiaquine plus sulfadoxine-pyrimethamine, loose <strong>combination</strong><br />
• AQ 10 mg/kg once daily <strong>for</strong> 3 days<br />
• SP 25/1.25 mg/kg once on the first day<br />
All doses supervized<br />
Outcomes 1. ACPR at day 28, PCR adjusted and unadjusted<br />
2. Gametocyte carriage at days 0, 7, 14, 21, and 28<br />
3. Mean increase in haemoglobin by day 28<br />
4. Adverse events<br />
Not included in the review:<br />
1. Fever clearance<br />
2. Parasite clearance<br />
Notes Country: Madagascar<br />
Setting: Primary health centres<br />
Transmission: Low and predominantly seasonal<br />
Resistance: CQ resistance<br />
Dates: Feb 2006 to June 2006<br />
Funding: Natixis, the Global Fund to Fight AIDS, Tuberculosis and Malaria, and the<br />
IAEA project<br />
Risk of bias<br />
Item Authors’ judgement Description<br />
Adequate sequence generation? Yes ’Randomization was in blocks of 5’. Drawing<br />
numbered papers from a box (additional<br />
detail from author).<br />
Allocation concealment? Yes ’Treatment regimens were allocated by an<br />
independent individual not involved in the<br />
analysis of the study’<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
79