Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
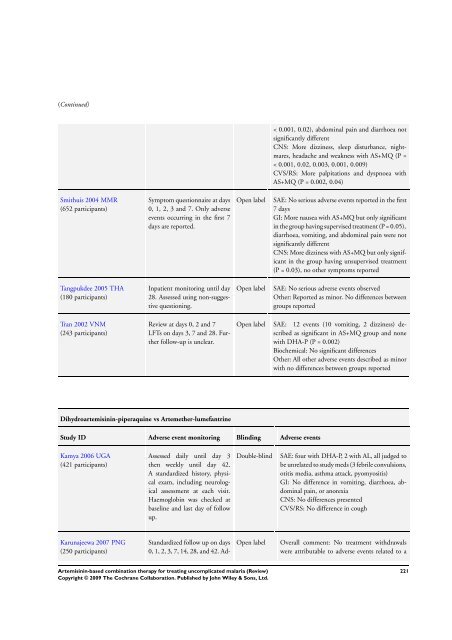
(Continued)<br />
Smithuis 2004 MMR<br />
(652 participants)<br />
Tangpukdee 2005 THA<br />
(180 participants)<br />
Tran 2002 VNM<br />
(243 participants)<br />
Symptom questionnaire at days<br />
0, 1, 2, 3 and 7. Only adverse<br />
events occurring in the first 7<br />
days are reported.<br />
Inpatient monitoring until day<br />
28. Assessed using non-suggestive<br />
questioning.<br />
Review at days 0, 2 and 7<br />
LFTs on days 3, 7 and 28. Further<br />
follow-up is unclear.<br />
Dihydroartemisinin-piperaquine vs Artemether-lumefantrine<br />
Study ID Adverse event monitoring Blinding Adverse events<br />
Kamya 2006 UGA<br />
(421 participants)<br />
Karunajeewa 2007 PNG<br />
(250 participants)<br />
Assessed daily until day 3<br />
then weekly until day 42.<br />
A standardized history, physical<br />
exam, including neurological<br />
assessment at each visit.<br />
Haemoglobin was checked at<br />
baseline and last day of follow<br />
up.<br />
Standardized follow up on days<br />
0, 1, 2, 3, 7, 14, 28, and 42. Ad-<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
< 0.001, 0.02), abdominal pain and diarrhoea not<br />
significantly different<br />
CNS: More dizziness, sleep disturbance, nightmares,<br />
headache and weakness with AS+MQ (P =<br />
< 0.001, 0.02, 0.003, 0.001, 0.009)<br />
CVS/RS: More palpitations and dyspnoea with<br />
AS+MQ (P = 0.002, 0.04)<br />
Open label SAE: No serious adverse events reported in the first<br />
7 days<br />
GI: More nausea with AS+MQ but only significant<br />
in the group having supervised treatment (P = 0.05),<br />
diarrhoea, vomiting, and abdominal pain were not<br />
significantly different<br />
CNS: More dizziness with AS+MQ but only significant<br />
in the group having unsupervised treatment<br />
(P = 0.03), no other symptoms reported<br />
Open label SAE: No serious adverse events observed<br />
Other: Reported as minor. No differences between<br />
groups reported<br />
Open label SAE: 12 events (10 vomiting, 2 dizziness) described<br />
as significant in AS+MQ group and none<br />
with DHA-P (P = 0.002)<br />
Biochemical: No significant differences<br />
Other: All other adverse events described as minor<br />
with no differences between groups reported<br />
Double-blind SAE: four with DHA-P, 2 with AL, all judged to<br />
be unrelated to study meds (3 febrile convulsions,<br />
otitis media, asthma attack, pyomyositis)<br />
GI: No difference in vomiting, diarrhoea, abdominal<br />
pain, or anorexia<br />
CNS: No differences presented<br />
CVS/RS: No difference in cough<br />
Open label Overall comment: No treatment withdrawals<br />
were attributable to adverse events related to a<br />
221