Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
5 No serious indirectness: Trials were conducted in a variety of African countries with variable transmission and resistance patterns.<br />
Children aged < four months and pregnant or lactating women were excluded.<br />
6 Serious imprecision: <strong>The</strong> 95% CI of the pooled estimate includes appreciable benefit with ASAQ over AL6 and crosses the line of no<br />
effect.<br />
7 No serious limitations: Allocation concealment was assessed as ’low risk of bias’ in five trials. Sensitivity analysis removing the trials<br />
with inadequate allocation concealment did not substantially alter the result.<br />
8 Very serious inconsistency: Heterogeneity was high so data were not pooled. This heterogeneity seemed to be related to region<br />
(with trials from East Africa favouring AL6 and trials from West Africa favouring ASAQ) and transmission intensity (with two trials<br />
experiencing very high rates of new infections).<br />
9 Very serious imprecision: Data were not pooled due to heterogeneity. <strong>The</strong> effect estimate is likely to vary between settings.<br />
10 Only one trial reported P. vivax and there were too few events to draw a conclusion.<br />
11 Dorsey 2006 UGA had adequate allocation concealment and blinding. In Faye 2003 SEN no allocation concealment or blinding<br />
was described.<br />
12 Very serious inconsistency: Heterogeneity was high so data were not pooled.<br />
13 Trials were conducted in Senegal (moderate transmission) and Uganda (mesoendemic).<br />
14 Very serious imprecision: <strong>The</strong> two trials reporting this outcome had very different results.<br />
15 Very serious imprecision: <strong>The</strong> 95% CI of the pooled estimate includes appreciable benefit and harm with each drug over the other.<br />
16 Serious limitations: Four out of five trials were unblinded.<br />
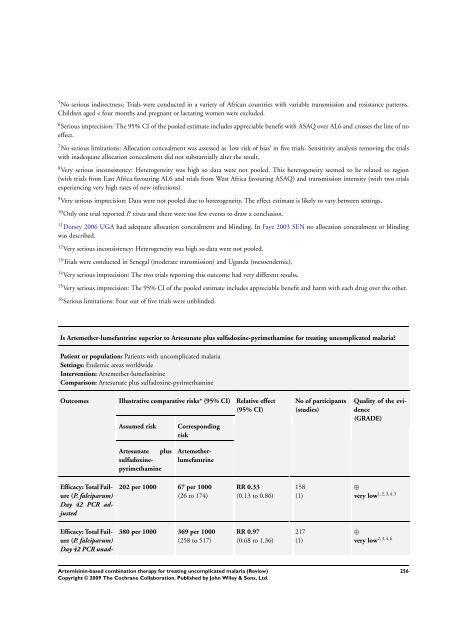
Is Artemether-lumefantrine superior to Artesunate plus sulfadoxine-pyrimethamine <strong>for</strong> treating uncomplicated malaria?<br />
Patient or population: Patients with uncomplicated malaria<br />
Settings: Endemic areas worldwide<br />
Intervention: Artemether-lumefantrine<br />
Comparison: Artesunate plus sulfadoxine-pyrimethamine<br />
Outcomes Illustrative comparative risks* (95% CI) Relative effect<br />
(95% CI)<br />
Efficacy: Total Failure<br />
(P. falciparum)<br />
Day 42 PCR adjusted<br />
Efficacy: Total Failure<br />
(P. falciparum)<br />
Day 42 PCR unad-<br />
Assumed risk Corresponding<br />
risk<br />
Artesunate plus<br />
sulfadoxinepyrimethamine<br />
Artemetherlumefantrine<br />
202 per 1000 67 per 1000<br />
(26 to 174)<br />
380 per 1000 369 per 1000<br />
(258 to 517)<br />
RR 0.33<br />
(0.13 to 0.86)<br />
RR 0.97<br />
(0.68 to 1.36)<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
No of participants<br />
(studies)<br />
158<br />
(1)<br />
217<br />
(1)<br />
Quality of the evidence<br />
(GRADE)<br />
⊕<br />
very low 1,2,3,4,5<br />
⊕<br />
very low 2,3,4,6<br />
256