Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
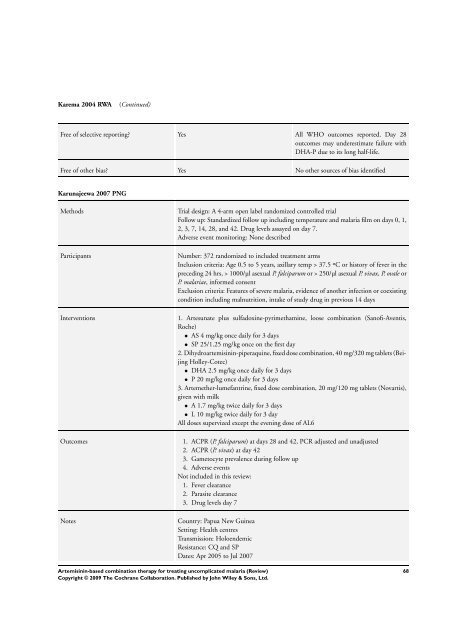
Karema 2004 RWA (Continued)<br />
Free of selective reporting? Yes All WHO outcomes reported. Day 28<br />
outcomes may underestimate failure with<br />
DHA-P due to its long half-life.<br />
Free of other bias? Yes No other sources of bias identified<br />
Karunajeewa 2007 PNG<br />
Methods Trial design: A 4-arm open label randomized controlled trial<br />
Follow up: Standardized follow up including temperature and malaria film on days 0, 1,<br />
2, 3, 7, 14, 28, and 42. Drug levels assayed on day 7.<br />
Adverse event monitoring: None described<br />
Participants Number: 372 randomized to included treatment arms<br />
Inclusion criteria: Age 0.5 to 5 years, axillary temp > 37.5 ºC or history of fever in the<br />
preceding 24 hrs, > 1000/µl asexual P. falciparum or > 250/µl asexual P. vivax, P. ovale or<br />
P. malariae, in<strong>for</strong>med consent<br />
Exclusion criteria: Features of severe malaria, evidence of another infection or coexisting<br />
condition including malnutrition, intake of study drug in previous 14 days<br />
Interventions 1. Artesunate plus sulfadoxine-pyrimethamine, loose <strong>combination</strong> (Sanofi-Aventis,<br />
Roche)<br />
• AS 4 mg/kg once daily <strong>for</strong> 3 days<br />
• SP 25/1.25 mg/kg once on the first day<br />
2. Dihydroartemisinin-piperaquine, fixed dose <strong>combination</strong>, 40 mg/320 mg tablets (Beijing<br />
Holley-Cotec)<br />
• DHA 2.5 mg/kg once daily <strong>for</strong> 3 days<br />
• P 20 mg/kg once daily <strong>for</strong> 3 days<br />
3. Artemether-lumefantrine, fixed dose <strong>combination</strong>, 20 mg/120 mg tablets (Novartis),<br />
given with milk<br />
• A 1.7 mg/kg twice daily <strong>for</strong> 3 days<br />
• L 10 mg/kg twice daily <strong>for</strong> 3 day<br />
All doses supervized except the evening dose of AL6<br />
Outcomes 1. ACPR (P. falciparum) at days 28 and 42, PCR adjusted and unadjusted<br />
2. ACPR (P. vivax) at day 42<br />
3. Gametocyte prevalence during follow up<br />
4. Adverse events<br />
Not included in this review:<br />
1. Fever clearance<br />
2. Parasite clearance<br />
3. Drug levels day 7<br />
Notes Country: Papua New Guinea<br />
Setting: Health centres<br />
Transmission: Holoendemic<br />
Resistance: CQ and SP<br />
Dates: Apr 2005 to Jul 2007<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
68